Exploring the Scales in Bioprocessing
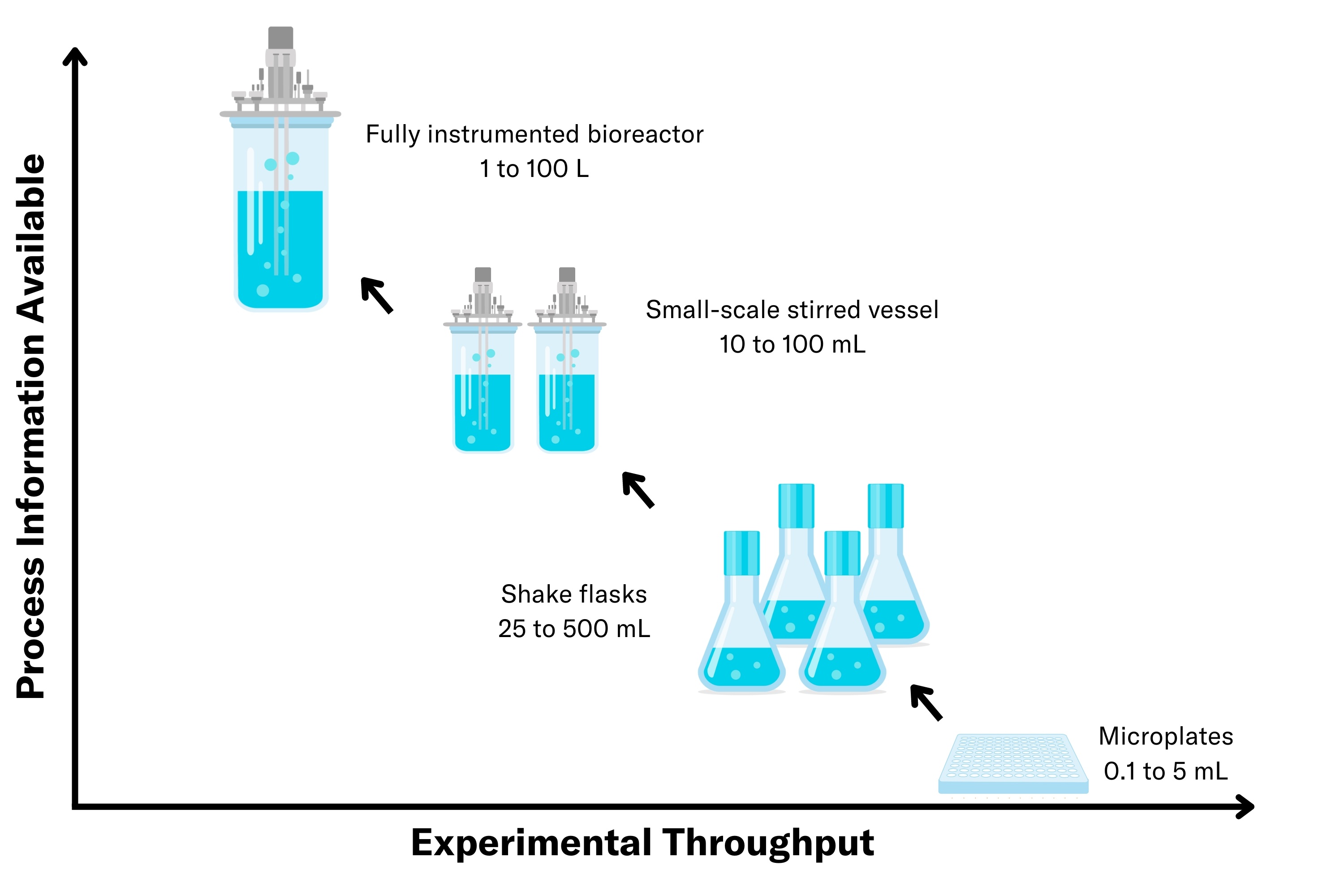
In bioprocessing, success often hinges on the ability to translate small-scale experiments into large-scale operations efficiently and effectively. Scaling is more than just increasing volume; it’s a complex process that requires balancing biological variability, engineering challenges, and regulatory requirements. From initial small-scale screenings to process development, scale-up, and ultimately full-scale production, each step demands careful consideration to maintain product quality, optimize yields, and ensure economic viability.
Microtiter Plates (MTPs)
Microtiter plates are used for initial high-throughput screenings at a micro/small scale (typically 100 µl to 10 ml). They can be used to test multiple conditions and clones/strains simultaneously, such as different media formulations, growth factors or process parameters like pH. Automated MTP systems can be used to handle multiple plates, allowing for rapid data collection and analysis of biomass concentrations, product yields and other relevant parameters, helping researchers identify optimal conditions for further scale-up. There also are more advanced microbioreactor systems that allow for continuous cultivations with active shaking and temperature control while generating data on cell growth, DO, fluorescence and pH. MTP systems can often be combined with liquid handling robots that can automate tasks such as media dispensing, sample handling and plate manipulation to further reduce hands-on time and by doing so, increase the throughput.
| Advantages | Disadvantages |
|
|
|
|
|
|
|
Shake Flasks
Shake flasks are used for small-scale cultivations under controlled conditions with typical working volumes of 10 ml to 1 l. Some researchers even conduct their initial screenings in parallelized shake flask experiments. But most often they are used to further characterize a pre-selected amount of targets even further. In contrast to MTPs, shake flasks allow for easy sampling for offline analysis of cell density, substrate concentration, metabolite production and other key parameters. Despite online sensors being available for many critical process parameters like biomass, DO and pH, the lack of data and automation is seen as a weakness of shake flasks.
| Advantages | Disadvantages |
|
|
|
|
|
|
|
|
|
|
|
Bioreactors
Stirred tank bioreactors are the main vessels used for scale-up and and production of biotech products, ranging from several liters (bench-top, glass) to thousands of liters in volume (stainless steel). Once optimized in microtiter plates and shake flasks, the process is scaled up to bioreactors for further process development and, in the end, production. Bioreactors ensure optimal oxygen supply through active aeration/gassing and stirring, proper nutrient supply by feeding with pumps (fed-batch) and pH control through titration with acids and bases throughout the whole cultivation. Sensors and control systems are used to monitor and adjust parameters like pH, temperature and dissolved oxygen to maintain optimal conditions for cell growth and product synthesis. More advanced scale-up optimizations may involve optimizing impeller designs, aeration strategies and feeding patterns.
| Advantages | Disadvantages |
|
|
|
|
|
|
|
Conclusion
In summary, the scale-up workflow in bioprocessing typically begins with proof-of-concept experiments in microtiter plates, where different strains and conditions are screened. The process then progresses to shake flasks, which are used to optimize key parameters and confirm scalability. Finally, it culminates in the transfer to stirred-tank bioreactors for advanced process development and production-scale testing, ensuring robustness, scalability, and reproducibility.
Each scale in this workflow comes with distinct advantages and limitations, and researchers strategically select the appropriate scale based on their specific needs, the stage of development, and production requirements. Among these vessels, the shake flask stands out as a versatile tool widely used across academia and industry. Its combination of high throughput, manageable volume, low cost, and ease of use makes it indispensable in bioprocessing workflows.