Data Spotlight
High-resolution Growth Curves Monitored With the Cell Growth Quantifier Reveal Toxicity of M-cresol on S. cerevisiae Cells
Background
M-cresol (3-methylphenol) holds significance in industrial applications as a flavor and platform chemical. It serves as a precursor for various substances, including menthol, and finds use in disinfectants and antiseptic agents due to its antibacterial and antifungal properties. In this study the biotechnological production of m-cresol from sugar utilizing the yeast Saccharomyces cerevisiae was achieved.
Considering the expected toxicity of m-cresol on the yeast cells involved in its production, a toxicity test was conducted with S. cerevisiae cells exposed to different concentrations of m-cresol.
Five distinct concentrations of m-cresol (150 mg/L, 300 mg/L, 450 mg/L, 600 mg/L, and 750 mg/L) were introduced into the yeast culture medium, alongside a negative control devoid of m-cresol (0 mg/L). Growth was monitored over a fermentation period of 144 hours using the Cell Growth Quantifier (CGQ). The high-resolution growth curves obtained from these parallel experiments clearly demonstrate the toxicity of m-cresol on yeast cells, with noticeable differences apparent among the various concentrations.
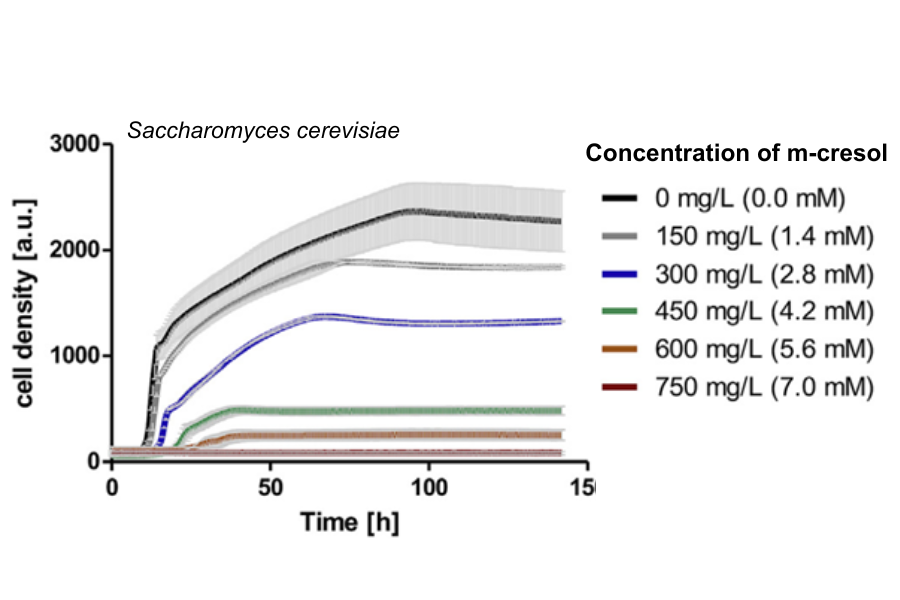
Toxicity test with m-cresol and S. cerevisiae wild type cells. The toxic effect of m-cresol is clearly visible by the diminished cell growth which is more apparent at higher concentrations (e.g., no growth detected at 750 mg/L) but already shows at the lowest concentration of 150 mg/L.
Cell densities (starting OD600 = 0.1) were followed over 144 h with the Cell Growth Quantifier and are depicted as arbitrary units (a.u.). Growth curves represent average of two biological replicates including standard deviations (light grey bars).

Materials & Methods
Precultures were prepared in 150 mL YPD (complex yeast medium with glucose) in 500 mL shake flasks and innoculated with S. cerevisiae cells to an optical density (OD600) of 0.1. These were shaken overnight at 180 rpm at 30°C.
50 mL YPD, respectively, was prepared in 300 mL shake flasks and supplemented with the respective concentration of m-cresol (0, 150, 300, 450, 600, or 750 mg/L - in replicates), and innoculated with cells from the overnight preculture to an OD600 of 0.1. Cell densities were closely monitored with the CGQ for the entire fermentation of 144 hours.
Conclusion
The performed toxicity test revealed that even at the lowest tested concentration of 150 mg/L, growth of the S. cerevisiae strain exhibited a slight reduction. Subsequently, growth rates were significantly diminished within the concentration range of 450–600 mg/L, and complete inhibition occurred at 750 mg/L of m-cresol. In summary, m-cresol demonstrates notable toxicity towards S. cerevisiae. This toxicity was effectively visualized through high-resolution growth curves using the Cell Growth Quantifier. The non-invasive, online measurement facilitated a streamlined and parallelized experiment involving 12 flasks (with 6 replicates of m-cresol concentrations), eliminating the need for manual sampling.
Source
Hitschler J. and Boles, E., 2019. De novo production of aromatic m-cresol in Saccharomyces cerevisiae mediated by heterologous polyketide synthases combined with a 6-methylsalicylic acid decarboxylase. Metabolic Engineering Communications. DOI: 10.1016/j.mec.2019.e00093
Want Results Like These?
We will work with you on a solution that works best for your application.
From Estimation To High-Resolution Growth Curves
Customer Success Stories
.png)
-Kitana Manivone Kaiphanliam (Washington State University)
