Data Spotlight
Improving Yeast Strain Robustness for Biorefinery Applications
Background
Interest in oil-based biochemical and biofuel production processes has increased because of increasing energy demand coupled with the rising concern of global warming. In the last decade, microbial biofuel production through economical bioprocesses has shown a high potential as an alternative to conventional ways of fuel production. The upstream processing includes the producer microorganism, which is the selection of a suitable microorganism in the preliminary phase of the fermentation. Wheat straw hydrolysate (WSH) is a type of lignocellulosic hydrolysate derived from wheat straw, an agricultural residue. It is abundant in renewable agricultural byproducts and potentially an excellent sustainable feedstock for biofuel and biochemical production. However, in WSH, several inhibitory compounds exist, hence demanding the development of more robust microbial strains that can tolerate the respective stressors and efficiently convert the sugars into the respective final product.
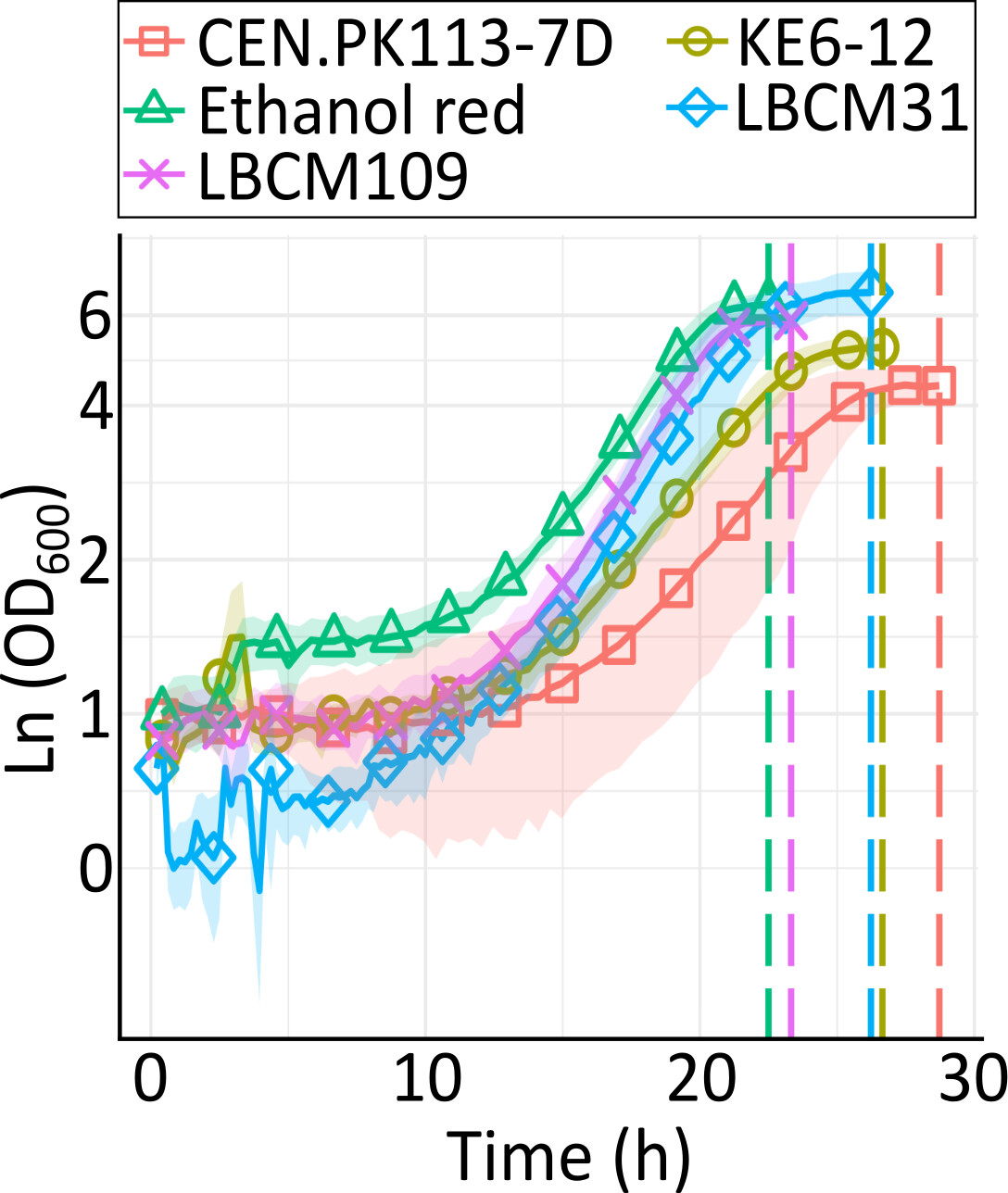
Anaerobic cultivation of five S. cerevisiae strains.
Materials & Methods
In this work, five S. cerevisiae strains were used: the laboratory strain (CEN.PK113-7D) two industrial strains (Ethanol Red and KE6-12,) and two wild-type strains. These yeasts were kept in a yeast extract peptone dextrose (YPD) medium of the following composition:10 g/L yeast extract, 20 g/L peptone, and 20 g/L glucose.
Growth experiments were performed in a minimal liquid medium supplemented with 70% (wt/wt) wheat straw hydrolysate (WSH). This was done with potassium phosphate, urea, magnesium phosphate, k-phthalate, trace metal solution, and vitamin solution. Sterilization of the medium takes place using 0.2 µm nylon membrane filters. pH version was adjusted to 5.5. The precultures were inoculated from glycerol cryostocks and incubated overnight at 30°C and 200 rpm in (YPD) medium. All strains grew similarly in (YPD) medium under the aerobic condition.
For the anaerobic culture, set up non-baffled shake flasks with a medium first gassed with N2, with an air trap filled with sterile glycerol to prevent oxygen diffusion. The conditions for cultivation of the cultures were 30°C, 200 rpm, while growth was monitored via the Cell Growth Quantifier (CGQ).
Conclusion
This study indicated that the five Saccharomyces cerevisiae grew similarly in wheat straw hydrolysate under anaerobic conditions but had extreme differences in transcriptional stress response.
The chosen strains allowed the differentially expressed genes to be directly compared under aerobic conditions in WSH without interference from physiology-driven confounding variables. However, it was the case that the activities of the different strains were very similar in growth pattern.
The key observations of this study were higher expression of genes related to glutathione metabolism, iron homeostasis, and membrane biosynthesis in wildtype isolates; some sugar transporter genes that were highly expressed in LBCM strains might reflect variation in evolutionary adaptations relative to the tolerance toward hydrolysate. The study provides insights into genetic targets for improving yeast strain robustness for biorefinery applications.
Source
Cámara E, Mormino M, Siewers V, Nygård Y.2024.Saccharomyces cerevisiae strains performing similarly during fermentation of lignocellulosic hydrolysates show pronounced differences in transcriptional stress responses. Appl Environ Microbiol90:e02330-23. https://doi.org/10.1128/aem.02330-23
From Estimation To High-Resolution Growth Curves
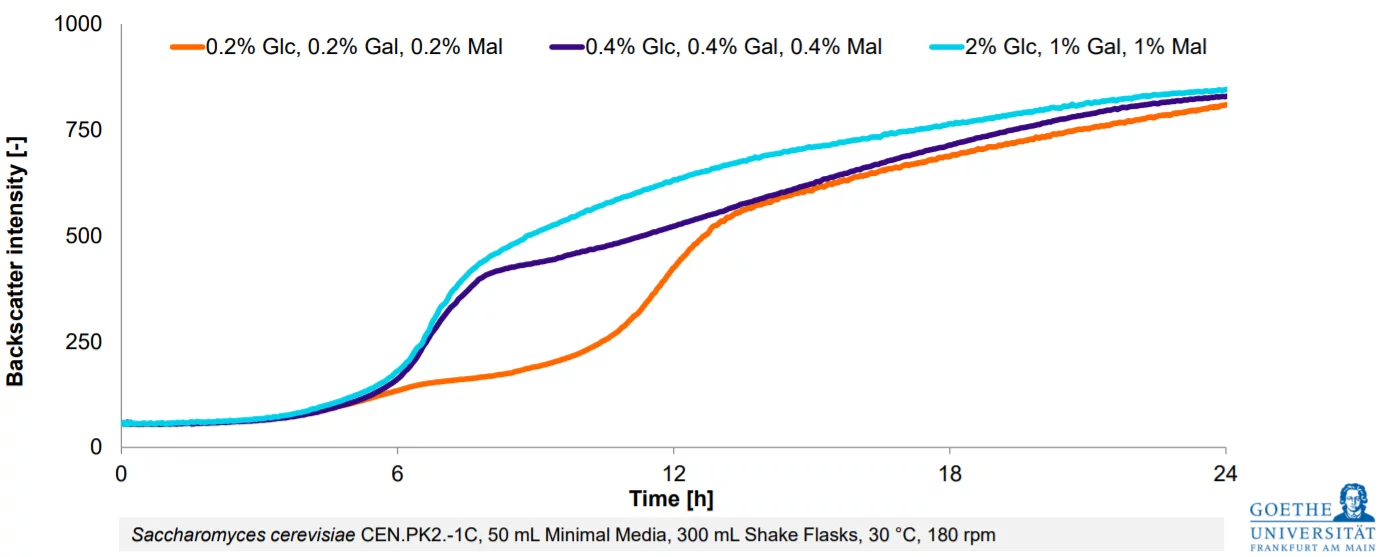
Customer Success Stories
.png)
-Kitana Manivone Kaiphanliam (Washington State University)
