Data Spotlight
Improved Biomass Monitoring in Bioreactors with the Mycel-forming Fungus Penicillium sp. in Repeated-batch Fermentations
Background
Filamentous fungi can grow in several different morphologies, e.g., in free mycelium or pellet form. In this study Penicillium sp., a well-known filamentous fungi species, was cultured in pellet form to produce a protease inhibitor that can inhibit the protease enzymes of Human African Trypanosomiasis or sleeping disease and is therefore used in efforts to contain this disease. This inhibitor is a secondary metabolite that is produced intracellularly at the end of the fermentation process. Hence, a repeated batch fermentation was implemented and pellets were harvested after 5-6 days.
To control the bioprocess, several parameters, including biomass were monitored. However, the identification of a suitable method for biomass monitoring posed a challenge. Many of the sensors available in the market are mostly not suitable for microorganisms that are not evenly distributed in the fermentation broth. Additionally, shear stress needed to be avoided to protect the integrity of the pellets. Therefore, introducing additional probes would have been suboptimal for this process.
The gravimetric method of bio dry weight determination was used which is time- and energy-consuming. To reduce this workload and enhance data density, the Cell Growth Quantifier BioR (CGQ BioR) was integrated. Its convenient attachment to the external glass wall of the bioreactor proved advantageous as it imposed no additional shear stress on the process.
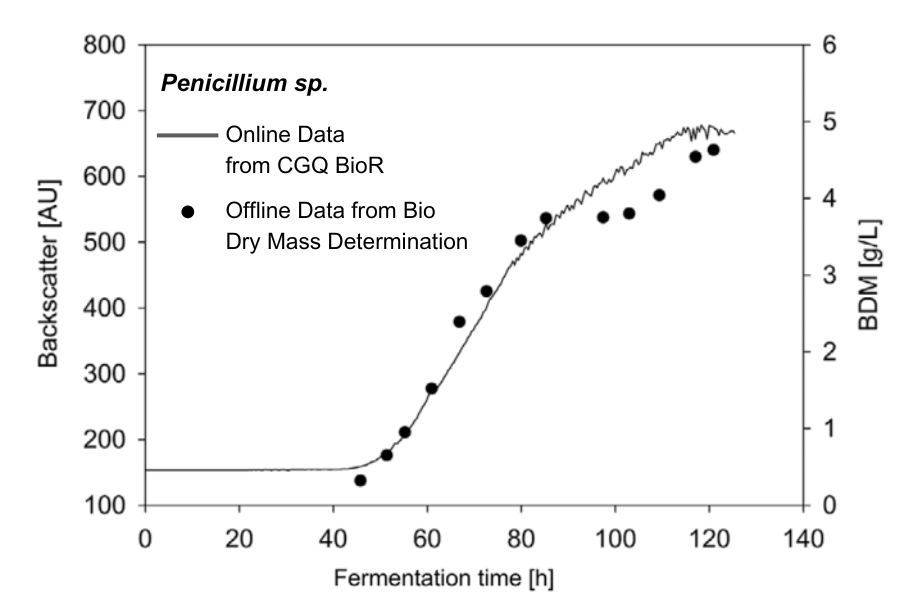
Growth profile of Penicillium sp. cells grown in pellet form. Growth was measured online with the CGQ BioR (continuous line) and offline via bio dry mass determination (dots). A very good correlation between the sensor data and the determined bio dry mass was observed.

Materials & Methods
A submerged cultivation of Penicillium sp. was performed in a 2 L double wall glass bioreactor. The cultivation lasted 5-6 days and was started by inoculating the medium with fungi spores. During the fermentation, dissolved oxygen (DO) in the medium was regulated: As soon as the DO-value droped below 30%, the atmospheric air flow was increased and if not sufficient, pure oxygen was provided.
To conduct a repeated-batch fermentation a sporulation support was implemented. During the batch fermentation the sporulation support captured some biomass. After the batch fermentation was completed, the bioreactor was cleaned-in-place so that all biomass, except the one from the sporulation support was removed. The captured biomass on the sporulation support then underwent sporulation in the next step, and the spores were used to start the next batch fermentation in fresh medium.
Conclusion
In this study, a long-term repeated-batch fermentation of the filamentous fungus Penicillium sp. in pellet form was designed in order to produce a protease inhibitor. A sporulation support was used to accumulate a suitable amount of biomass that remains in the bioreactor even during intermediate cleaning steps, to support fungal growth in the form of pellets. The CGQ BioR was a valuable addition to the automated fermentation as it is non-invasive and therefore did not introduce additional shear stress which would have hurt the fungal morphology and ultimately the growth of the fungus. Furthermore, CGQ BioR-derived data showed a very good correlation to data received from bio dry mass determination and is thus a valuable alternative to this work-intensive and invasive method that necessitates the usage of larger fermentation volumes. The automatic CGQ BioR measurement proceeds during nights and weekends, allowing for a high data density even at times where the laboratory is not occupied. Overall, the CGQ BioR sensor facilitated biomass monitoring in this process and improved the data quality.
Source
Soerjawinata, W., et al., 2022. Novel bioreactor internals for the cultivation of spore-forming fungi in pellet form, https://doi.org/10.1002/elsc.202100094
Read the interview with the author here!
Want Results Like These?
We will work with you on a solution that works best for your application.
From Estimation To High-Resolution Growth Curves


Customer Success Stories
.png)
-Kitana Manivone Kaiphanliam (Washington State University)
