Shaking
Speed
OTR increases with shaking speed.
Many microorganisms require oxygen as it is a necessary component in cellular processes, including respiration. However, oxygen needs can differ between organisms, and even between different strains. Oxygen is often considered a limiting factor in bioprocessing because if it falls below certain thresholds it can result in slowed metabolism, altered product formation, changed growth behavior, and in worst case, cell death.
Understanding and controlling dissolved oxygen levels is a key consideration for bioprocess engineers and scientists, as it can significantly impact the overall success of a bioprocessing operation.
The term 'dissolved oxygen' refers to the level of free, non-compound oxygen ions present in an aqueous solution. In bioprocessing, the presence of dissolved oxygen is vital for the growth and metabolism of aerobic microorganisms, as it serves as a crucial substrate for their energy production. DO is typically measured and reported as milligrams per liter [mg-DO/L of water (mg/L)], percent saturation (%), or partial pressure (pO2).
The DO concentration in a bioprocess is influenced by various factors, including temperature, pressure, and the rate of oxygen consumption by the organisms. As with pH, which is the measure of acidity or alkalinity in a solution, dissolved oxygen is an essential parameter that needs to be carefully monitored and controlled to optimize bioprocess outcomes.

While there are many variables that are important to cellular growth, oxygen is by far one of the most Critical Process Parameters (CPPs) in bioprocessing. Microorganisms can be divided into three main groups based on their oxygen requirements:
1. Obligate aerobes can produce energy only via aerobic respiration and require molecular oxygen to live.
2. Obligate anaerobes cannot tolerate any molecular oxygen and they require an oxygen-free environment to grow.
3. Facultative anaerobes on the other hand, can grow in both environments – in the presence of molecular oxygen and in its absence. Within the group of facultative anaerobes, two subgroups can be defined.
4. Aerotolerant organisms can grow in the presence of molecular oxygen, but they cannot use it. They would always produce their energy via anaerobic fermentation. Lactobacilli are prominent representatives of aerotolerant organisms.
5. The second subgroup of facultative anaerobic organisms, including many yeast species, would use oxygen for energy production if present and switch to the anaerobic fermentation when oxygen is depleted.
In addition, microaerobic organisms are organisms that need molecular oxygen to preserve energy (making them obligate aerobes), but they cannot tolerate the partial pressure of oxygen of the atmosphere. These organisms require lower oxygen partial pressures to survive.
Efficient oxygen transfer is essential for a successful aerobic cultivation. Regardless of vessel type or application, determining the volumetric oxygen transfer coefficient (kLa) and the oxygen transfer rate (OTR) can offer some important benefits, including:
The volumetric mass transfer coefficient (kLa) refers to the pace at which oxygen can move between the gas phase to the liquid phase. Consider a gas bubble containing oxygen in liquid. The kLa, can be represented by an equation.
kLa = kL × a
kL = mass transfer coefficient, describes the rate of molecular diffusion through the gas-liquid interface
a = the surface area available for diffusion
In most bioprocesses the most important step is the OTR, hence engineers and scientists try to affect it by changing their kLa.
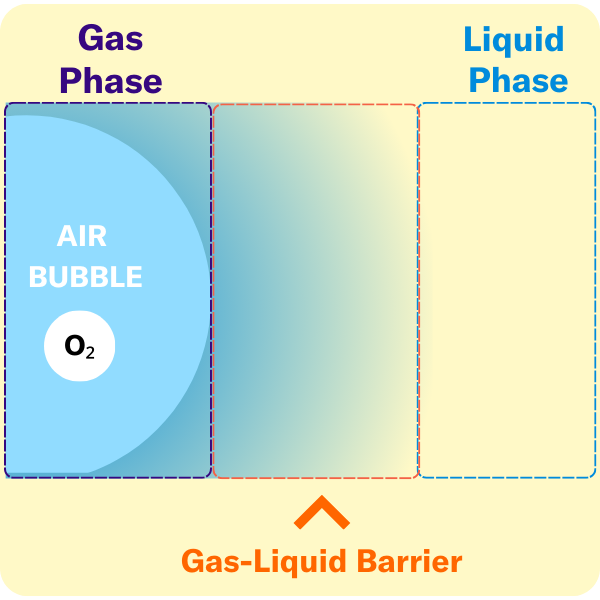
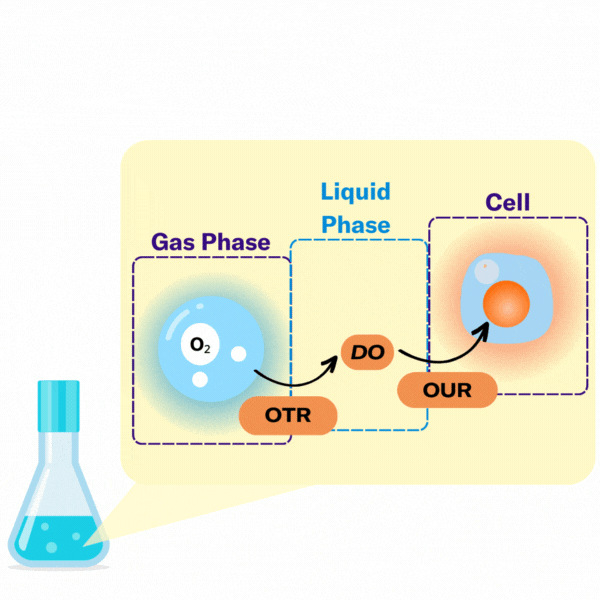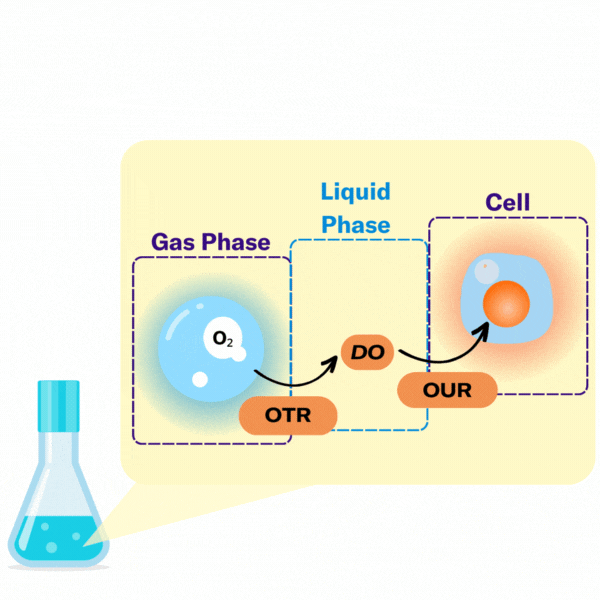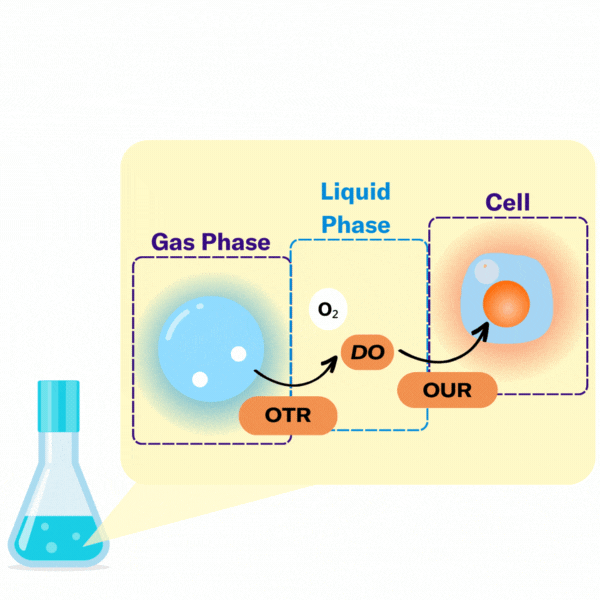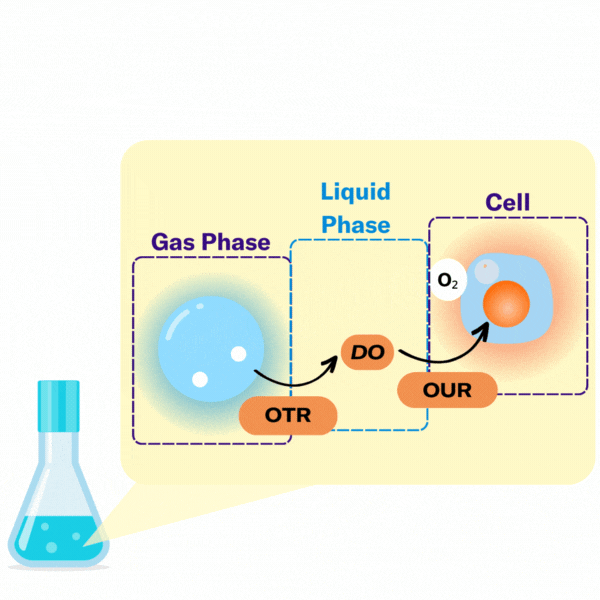
There are several key terms used to describe the points at which oxygen transfer occurs in bioprocessing.
When working with bioreactors, engineers use many different means to influence the kLa for a better OTR. This most often includes increasing small bubbles as well as pressure. Increasing OTR in shake flasks can be influenced by several factors.
In shake flasks, oxygen transfer from the headspace to the cells is not driven by bubbles but by the thickness of the liquid film.
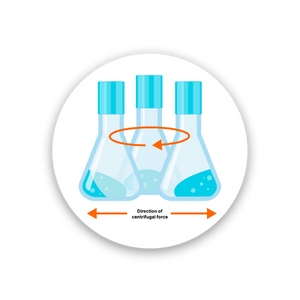
OTR increases with shaking speed.
Lower filling volumes result in higher OTR.

Higher viscosities will have lower OTR.
Baffles can be used to increase aeration.

Some closure types allow for more oxygen in the vessel than others.
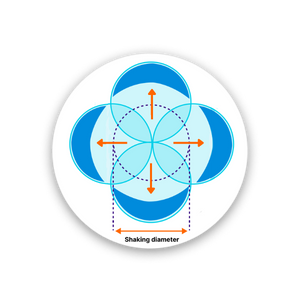
A larger shaking diameter can increase the mixing and aeration of the liquid.
All cells have an optimal pH level that supports their growth. While this can vary some, most cells prefer to be around neutral. When pH levels fall outside of the acceptable range, cell culture growth can become inhibited or altered and the titer of their products reduced. pH levels that are too acidic or alkaline can actually destabilize the genetic material of cells, which can cause mutations and ultimately cell death if not corrected.
It is not possible to visualize contamination in clear media, however, sudden changes in pH are a good indicator of bacterial or fungal contamination. Most cases of bacterial contamination in the cell culture laboratory are caused by aerobes.
When working with indicator dyes, the media will become acidic and appear yellow if contaminated with aerobic bacteria. However, if the bacteria are anaerobic, the contamination will cause the medium to become basic and will appear pink. A fungal contamination will cause the media to turn hazy.
Contamination is a concern because bacteria often produce toxins that disrupt cell function and can ultimately destroy cell cultures. Cell cultures are especially susceptible to contaminations because they grow much slower than most bacteria and fungi, so the latter easily overgrow mammalian cells in nutrient rich media.
.png?width=2352&height=2429&name=pH-Flasks-Yellow-and-Pink-Medium%20(1).png)
Culture media, also known as growth media, is any solution comprised of amino acids, vitamins, inorganic salts, glucose, and other substrates that support the health and growth of cells in culture. Many also contain serums as a source of growth factors, hormones, and attachment factors.
Depending on the cell type and preferred outcome, nutrient supplementation in cell culture can be used to increase cell viability and genomic stability. The surrounding pH can influence how cells take in these nutrients. Macronutrients such as nitrogen, potassium, calcium, magnesium, and sulfur are more readily available at pH 6.0–6.5, while micronutrients become less available at higher, alkaline levels of greater that 7.0.
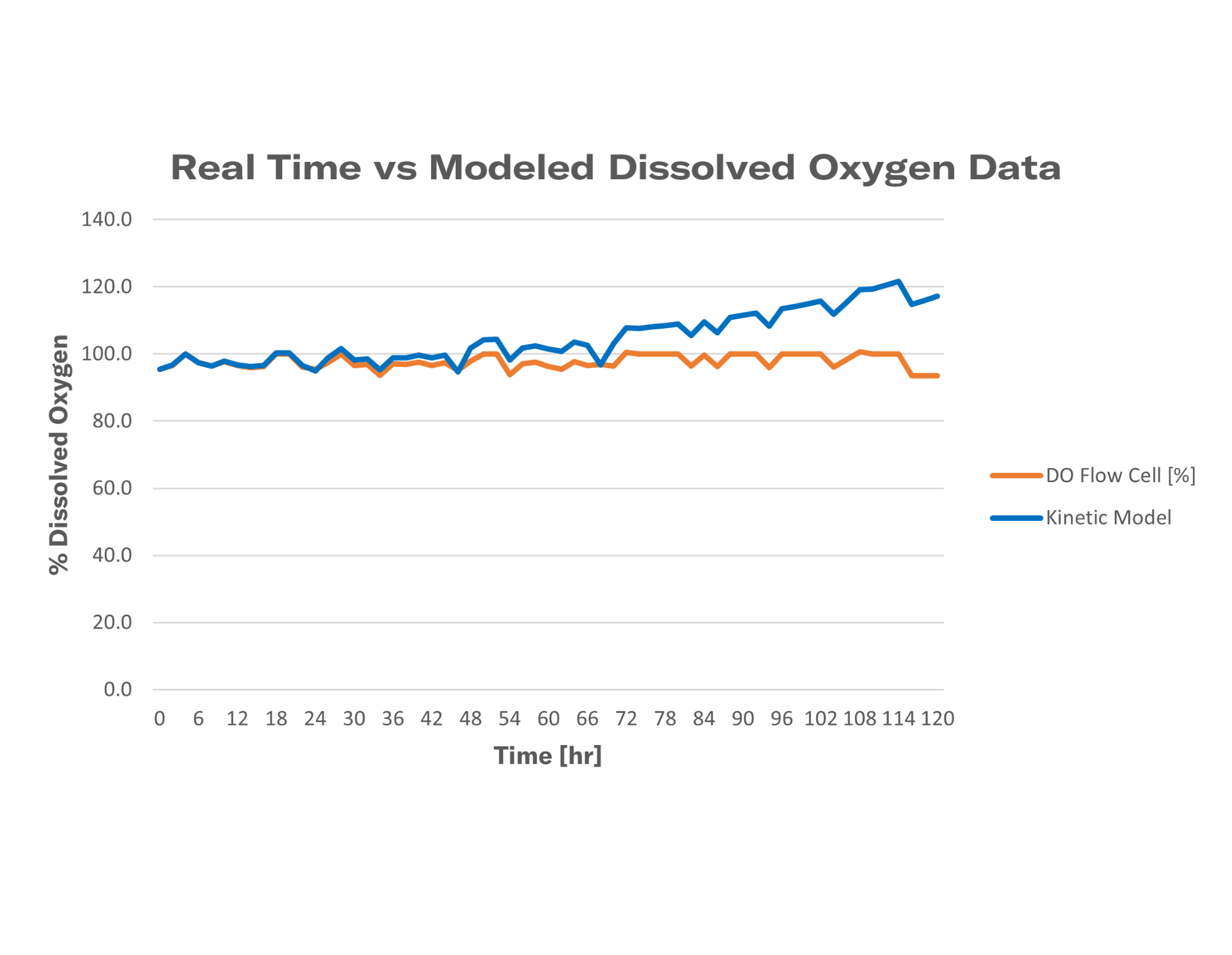
Oxygen limitations occur when the amount of available molecular oxygen falls below a certain threshold. Without close monitoring, shake flasks can often fall victim to oxygen limitations. While you cannot visually identify oxygen limitations, you can see the effects they have on your fermentation. The best options to identify oxygen limitations in cultivations include biomass and dissolved oxygen monitoring.
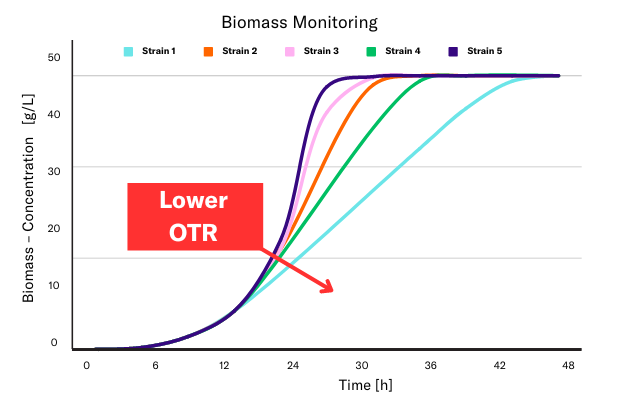
Lack of oxygen results in slowed growth of microorganisms, often associated with a lack of an exponential phase. While a linear curve does not correlate solely to oxygen limitation, secondary parameters can assist in determining the root cause.
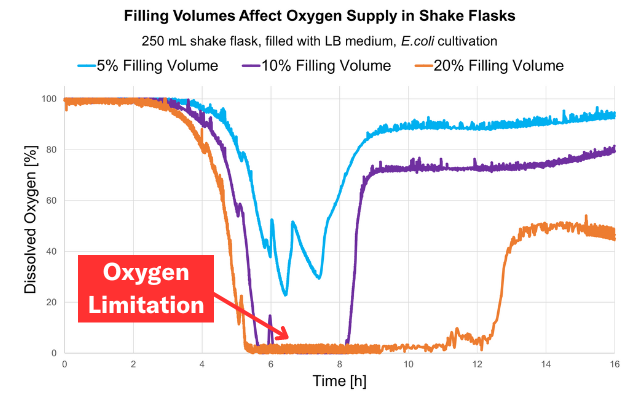
The organism utilizes oxygen to metabolize key nutrients. Without the addition of new resources, the levels of DO fall below the organism's threshold resulting in a limitation.

Available in different form factors (patches, integrated in flow cells, pills) for continuous measurements in small vessels.
Learn More About DO Sensor Pills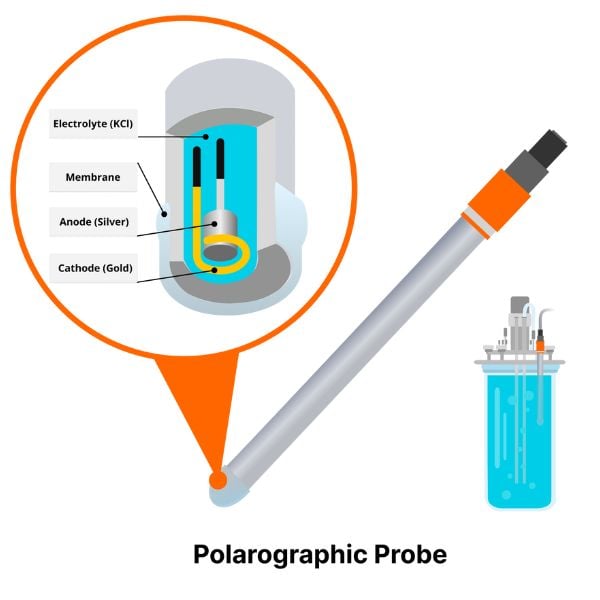
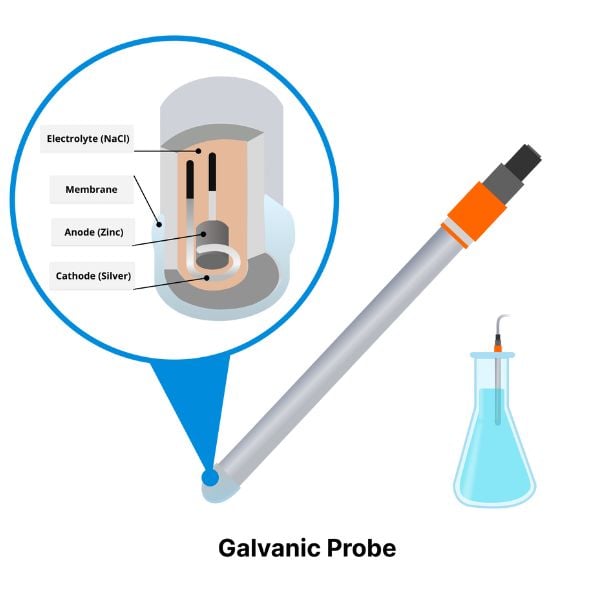

Available in different form factors (patches, integrated in flow cells, pills) for continuous measurements in small vessels.
Learn More About DO Sensor Pills


Novel, patented pill technology
Single-use pill: Factory-calibrated and pre-sterilized for immediate use
Drop & Go: Easy handling and fast experiment setup
Combine with LIS for DO-based feeding in shake flasks

Continuously monitor DO in flow loops using fiber optic sensors.
DO range: 0-50% O2 (gas), 0-100% O2 (liquid)
Factory-calibrated and pre-sterilized
Single-use design reduces risk of contamination