Bioprocess
- For a broad range of organisms and CHO cell types
- For most media compositions
The MPS is an optical sensor technology for the monitoring of multiple parameters in shake flasks.
-1.png?width=300&height=115&name=Part-of-DOTS%20(1)-1.png)
.png?width=285&height=285&name=for_microbial_and_cell_culture_banner%20(2).png)
As the central piece of sbi’s DOTS Platform, the MPS enables effortless online monitoring of multiple parameters in shake flasks. From dissolved oxygen (DO) and biomass monitoring to fluorescence detection – the MPS turns the standard shake flask into smart bioreactors.
Key Features
Benefits
For a broad range of shaking speeds and filling volumes
Bioprocess characterization
Cell line/Strain development, characterization, and selection
Collect critical process parameters for an informed upscaling process
Optimize process conditions with parameter-based feeding
.jpg)
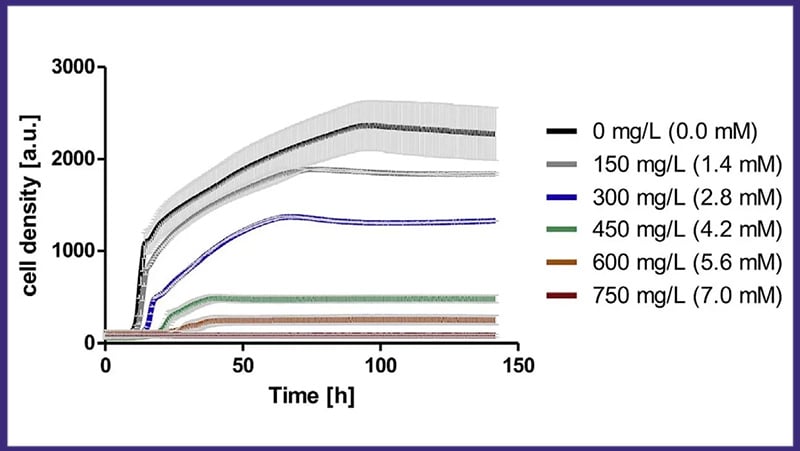
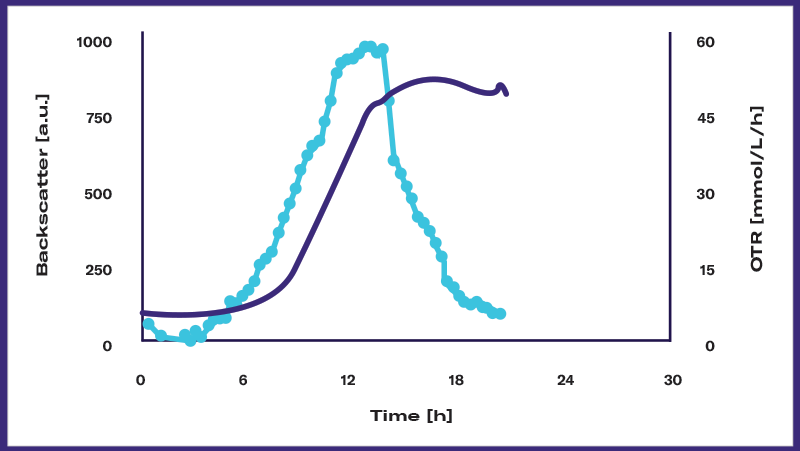
Escherichia coli (E. coli), 200 rpm/25 mm, 10 % filling volume, 250 ml flask, 37°C
DOTS enables real-time monitoring of multiple parameters. Early IPTG inoculation (marked by the red triangle) is used to induce protein expression, showing that GFP expression is growth-coupled. By continuously monitoring these parameters online, researchers can gain insights into the dynamics of metabolic events occurring within the shake flask culture.
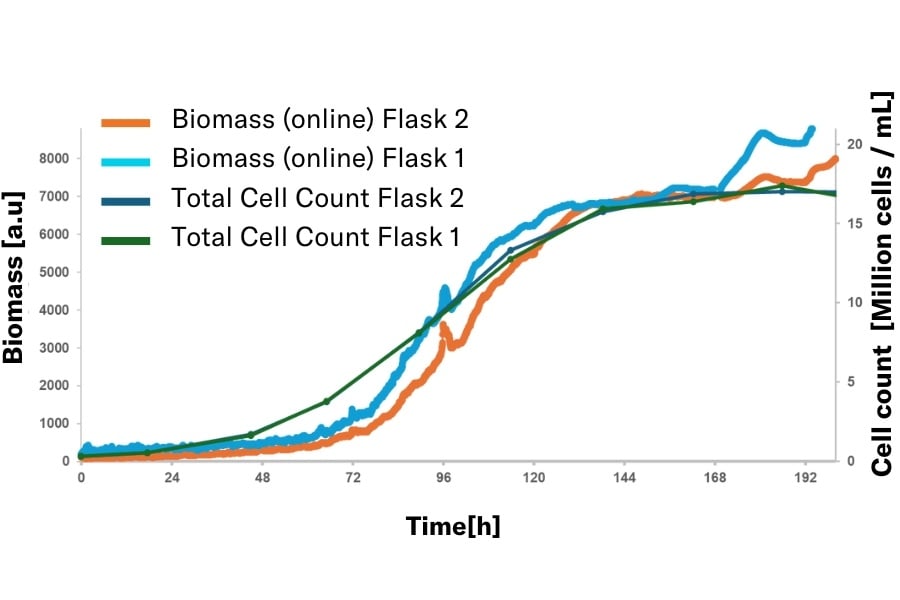
CHO Cell, 0.3 million cells / mL, Nalgene flask, 250 mL, 20% filling volume (50 mL), 150 rpm, 50 mm
Biomass for CHO cells demonstrates strong correlation with offline biomass measurements obtained via Total Cell Density (TCD) analysis. The MPS exhibits exceptional reproducibility when comparing data collected from two independent sensors (Flask 1 vs Flask 2), showcasing its consistency and robustness for process monitoring in bioprocess applications.
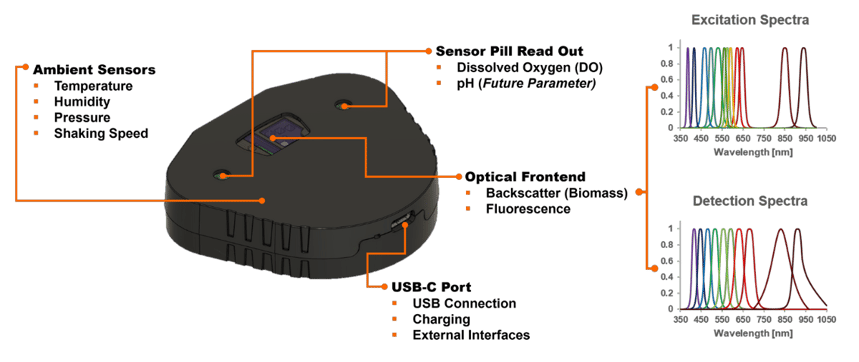
Each sensor plate is positioned in the adapter under the cultivation vessel and measures non-invasively through the vessel wall.
Several sensor plates can be connected to a single hub. The hub bundles the data from all monitored flasks and sends it to the DOTS Software.
The DOTS Software receives the data from the hub and visualizes relevant parameter data in real-time.

Darkening the shaker is highly recommended. The MPS has ambient light compensation but especially during fluorescence measurement, the signal is recorded over a period of several seconds, which is why external light can influence the measurement. For biomass measurement, the recording time is significantly shorter, but darkening can lead to better sensitivity (in particular in the lower OD ranges).
The biomass data is depicted in arbitrary units (a.u.) as backscatter signal. This provides a growth curve that contains all the information you would typically obtain from an OD₆₀₀ growth curve – but with much higher data density. However, if you specifically need your data expressed as OD₆₀₀ values, it is possible to take manual measurements at the beginning and end of the cultivation and use them to establish a correlation curve. This can only be done in post-processing, and the results will generally be less precise than a curve generated from a large number of offline OD₆₀₀ measurements.
As with any optical method, particles, color changes, or increased turbidity can affect the measurement. Anything that scatters or absorbs light may influence the backscatter signal. The extent of this effect depends on the concentration of particles and the degree of cloudiness. In many cases, if particle levels remain stable and the medium is not too turbid so that light can still pass through, reliable biomass measurements are still possible. Each situation should be evaluated individually. If you have doubts if your medium will work, please contact our support team.
No. The sensor relies on a stable shaken environment and cannot be used for a static culture.
The MPS can reliably measure biomass from an OD600 of 0.5 and a cell culture concentration of 1 Mio cells/mL. Depending on the process these values might deviate slightly. Below this concentration the graph will stay linear even if cells started to multiply.
No. The sensor is not water-proof and should always be kept in a dry environment. Splashing water from a culture, e.g., from higher shaking speeds, especially when using high filling volumes and/or baffles, should be avoided under any circumstances.
The liquid moves in a circle when shaken. This movement is divided into so-called bins and a measurement is taken in each of these 32 bins. Depending on the bioprocess conditions, the bin should be selected in which the liquid has the best position above the biomass measurement window. Our default bin is 17. If you would like to optimize the binning settings, please contact our support team. The team will be happy to help you choose the right bin.
Our default wavelength is 940 nm, which is usually well-suited for all common bioprocesses. By using an LED in the near-infrared range, other media components are excited as little as possible, resulting in minimal interference with the actual backscatter measurement. However, for some bioprocesses, a different wavelength may be useful. If you have any questions, please contact our support team.
Darkening the shaker is highly recommended. The MPS has ambient light compensation but especially during fluorescence measurement, the signal is recorded over a period of several seconds, which is why external light can influence the measurement. For biomass measurement, the recording time is significantly shorter, but darkening can lead to better sensitivity (in particular in the lower OD ranges).
The biomass data is depicted in arbitrary units (a.u.) as backscatter signal. This provides a growth curve that contains all the information you would typically obtain from an OD₆₀₀ growth curve – but with much higher data density. However, if you specifically need your data expressed as OD₆₀₀ values, it is possible to take manual measurements at the beginning and end of the cultivation and use them to establish a correlation curve. This can only be done in post-processing, and the results will generally be less precise than a curve generated from a large number of offline OD₆₀₀ measurements.
As with any optical method, particles, color changes, or increased turbidity can affect the measurement. Anything that scatters or absorbs light may influence the backscatter signal. The extent of this effect depends on the concentration of particles and the degree of cloudiness. In many cases, if particle levels remain stable and the medium is not too turbid so that light can still pass through, reliable biomass measurements are still possible. Each situation should be evaluated individually. If you have doubts if your medium will work, please contact our support team.
No. The sensor relies on a stable shaken environment and cannot be used for a static culture.
The MPS can reliably measure biomass from an OD600 of 0.5 and a cell culture concentration of 1 Mio cells/mL. Depending on the process these values might deviate slightly. Below this concentration the graph will stay linear even if cells started to multiply.
No. The sensor is not water-proof and should always be kept in a dry environment. Splashing water from a culture, e.g., from higher shaking speeds, especially when using high filling volumes and/or baffles, should be avoided under any circumstances.
The liquid moves in a circle when shaken. This movement is divided into so-called bins and a measurement is taken in each of these 32 bins. Depending on the bioprocess conditions, the bin should be selected in which the liquid has the best position above the biomass measurement window. Our default bin is 17. If you would like to optimize the binning settings, please contact our support team. The team will be happy to help you choose the right bin.
Our default wavelength is 940 nm, which is usually well-suited for all common bioprocesses. By using an LED in the near-infrared range, other media components are excited as little as possible, resulting in minimal interference with the actual backscatter measurement. However, for some bioprocesses, a different wavelength may be useful. If you have any questions, please contact our support team.
Manual sampling-based data is often not sufficient to fully understand the bioprocess. Offline sampling is complex and time consuming, resulting in lower measurement frequency with most pulls being at the start or the end of the experiment. This means that critical information from your growth phases are being overlooked, and could have a detrimental impact on your final product. Automated online measurements, on the other hand, never miss a moment. With a high resolution growth curve, you can detect bioprocess changes in real-time.