Overview

Key Facts
Department: Voiland School, Washington State University (WSU)
Research focus: Chemical Engineering and Bioengineering
Cell Line: Bovine CD8
cytotoxic T lymphocyte (CTL) infected with protozoan parasite, Theileria
parva
Advancing The Boundaries of Biotech
Students at the Voiland School of Chemical Engineering and Bioengineering, part of the Washington State University (WSU), create innovative solutions to address critical needs for today's society. Programs focus on three key research areas:
- Catalysis and kinetics
- Molecular and cellular engineering
- Biomechanics
Under the guidance of Professor Bernard Van Wie, researchers explore biosensors and bioanalytical devices in cell and tissue cultures, as well as making engineering education more accessible.
The Challenge
Cancer, a leading cause of death around the world, remains a significant issue in medicine. While immunotherapy treatments such as Chimeric Antigen Receptor T cell (CAR-T) therapies are becoming a more promising option because of their effectiveness in killing cancer cells without harming healthy tissue in the body, available technologies come at a high cost- a result of inefficient cell expansion and manufacturing methods.
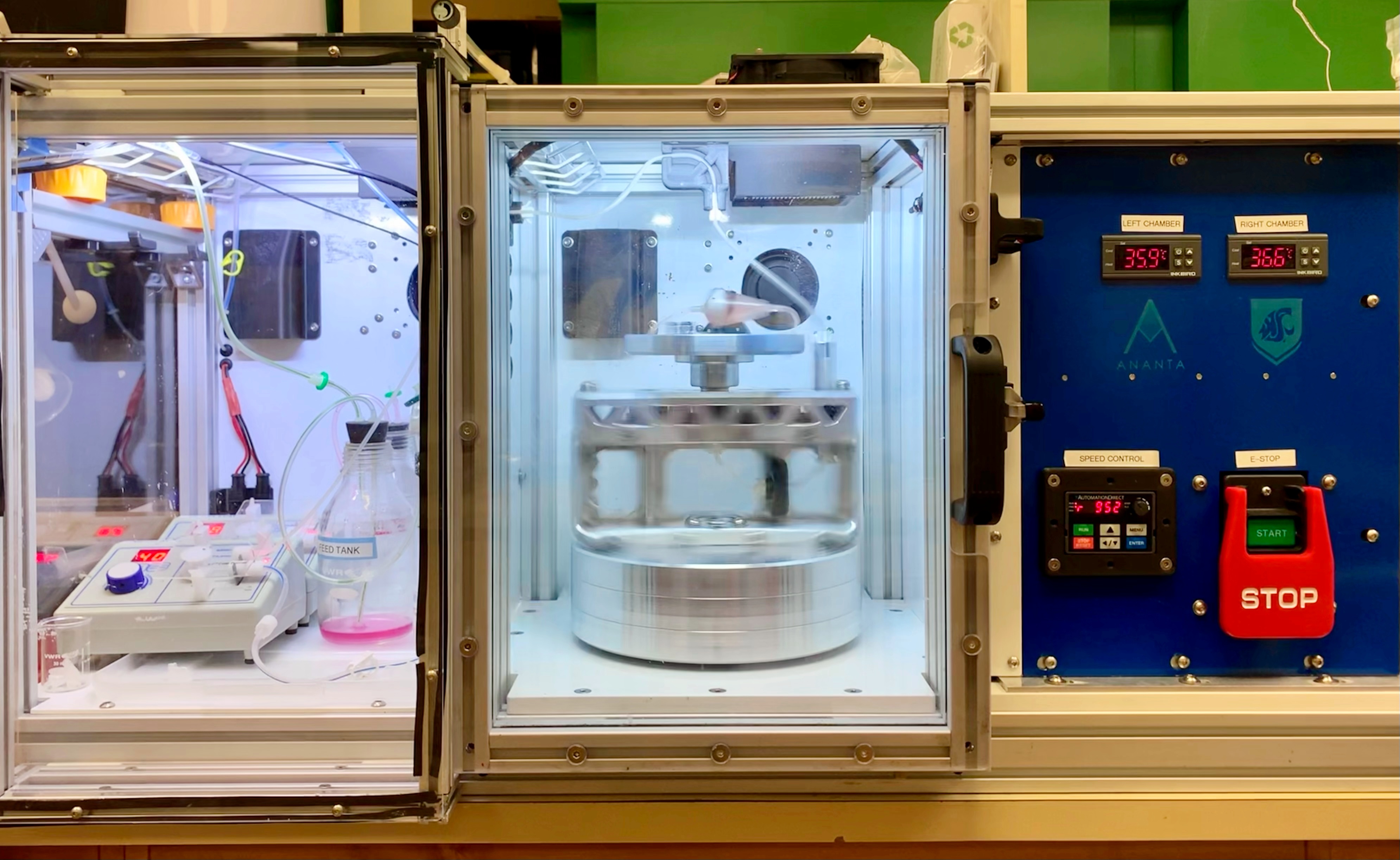
Researchers at Washington State University (WSU), Kitana Kaiphanliam and Brenden Fraser-Hevlin, developed a centrifugal bioreactor (CBR) as a potential answer to this manufacturing bottleneck.
Static batch culture experiments were run to determine growth parameters for a kinetic model designed to optimize cell growth in the CBR. In particular, researchers observed the response of the CTL cells to environmental changes, such as consumption of glucose and dissolved oxygen (DO), and to the production of toxic metabolites, including lactate and ammonium. It was through these earlier tests that dissolved oxygen was identified as a limiting factor.
Oxygen consumption rate (OCR) experiments were conducted and compared to literature values to determine which recycle rates through the oxygenator were required to keep DO at sufficient levels to maintain growth in the CBR over the 5-10 day expansion study. DO was measured with a portable oxygen meter at the beginning and end of the experiment, and cells were counted manually.
Challenges of Manual Dissolved Oxygen Sampling
Low-resolution data
![]() Time-consuming
Time-consuming
![]() Invasive sampling methods/disturbed bioprocess
Invasive sampling methods/disturbed bioprocess
![]() Increased risk of contamination
Increased risk of contamination
![]() Increase in human error
Increase in human error
Results
Dissolved oxygen data collected via the fiber optic sensors reported approximately 5% higher, on average, than the values predicted by the kinetic model (based on OCR from static culture studies). This finding highlighted that the cells are not consuming oxygen as fast as originally thought, and that perhaps the centrifugal forces or fluid forces occurring in bioreactor are affecting how the cells are consuming oxygen.

The Conclusion
Considered a critical process parameter in cell and tissue culture, dissolved oxygen plays a crucial role in the growth, metabolism, and viability of cells. The appropriate oxygen level can vary depending on the specific cell type, culture conditions, and production goals.
For the purpose of this study, it was essential to monitor the DO levels in the system to ensure that the cells had enough oxygen to grow. Moreover, it is imperative when working with T Cells to reduce the chance for cell exhaustion, a state of dysfunction that occurs as T Cells are exposed to chronic antigenic stimulation. As a result, the cells may become less responsive and less effective at eliminating the pathogen or cancer cells.
Incorporating an automated, non-invasive technology for dissolved oxygen gave researchers at WSU the ability to not only validate their kinetic model, but also provided new insights into the real-time oxygen consumption of their cells. This information allowed them to better understand T cell growth in a centrifugal bioreactor, and make changes to the CBR prototype in order to expand clinically relevant numbers of CTLs.
Want Results Like These?
We will work with you on a solution that works best for your application.
Testimonial
“Incorporating sbi’s DO flow cells into our system removed the need for manual sampling, saving us time, reducing the risk of contamination, and providing information on how the cells are growing even when we are not in the lab. With availability of this more detailed view of our culture, we can make informed improvements to our cell expansion process.”
-Dr. Kitana Manivone Kaiphanliam (Washington State University)
From Estimation To High-Resolution Growth Curves


Customer Success Stories
.png)
-Kitana Manivone Kaiphanliam (Washington State University)

Journal Reference:
- Kitana M. Kaiphanliam, Brenden Fraser‐Hevlin, Eric S. Barrow, William C. Davis, Bernard J. Van Wie. Development of a centrifugal bioreactor for rapid expansion of CD8 cytotoxic T cells for use in cancer immunotherapy. Biotechnology Progress, 2023; DOI: 10.1002/btpr.3388
