Data Spotlight
Dissolved oxygen measurements of filamentous fungi (A. oryzae) cultures reveal effects of different shaking speeds
Background
Aspergillus oryzae is a filamentous fungi or mold. It is also known as kōji mold and has been used in traditional culinary industries, including for food fermentation, brewing, and flavoring. Recently, A. oryzae is increasingly being used for the production of enzymes and heterologous proteins, due to beneficial characteristics such as a robust capacity for protein synthesis, secretion, and posttranslational modifications.
This study, conducted at Hochschule Niederrhein, working group of Prof. Dr. Michaela Wagner, focused on optimizing process conditions to enhance protein expression in a genetically modified A. oryzae strain. Notably, protein synthesis occurs in tandem with biomass formation, with higher biomass production correlating to increased protein yield. Oxygen availability takes on a critical role in this process, with protein expression being oxygen-dependent, underscoring the significance of adequate oxygen supply.
Elevating the shaking frequency of A. oryzae cultures in shake flasks typically enhances oxygen availability. To assess this, three distinct shaking speeds (300, 350, and 400 rpm) were applied, with dissolved oxygen levels measured using DO Sensor Pills and the Multiparameter Sensor (MPS).
Simultaneously, the Multiparameter Sensor monitored a biomass signal and the morphology of the mycelium aggregates was visually checked and aggregate sizes were measured. Higher shaking speeds resulted in smaller fungal aggregates, thereby increasing oxygen delivery to cells due to an increased surface-to-volume ratio. These morphological alterations likely influenced the backscatter signal recorded with the MPS, potentially accounting for the observed decrease in biomass signal.
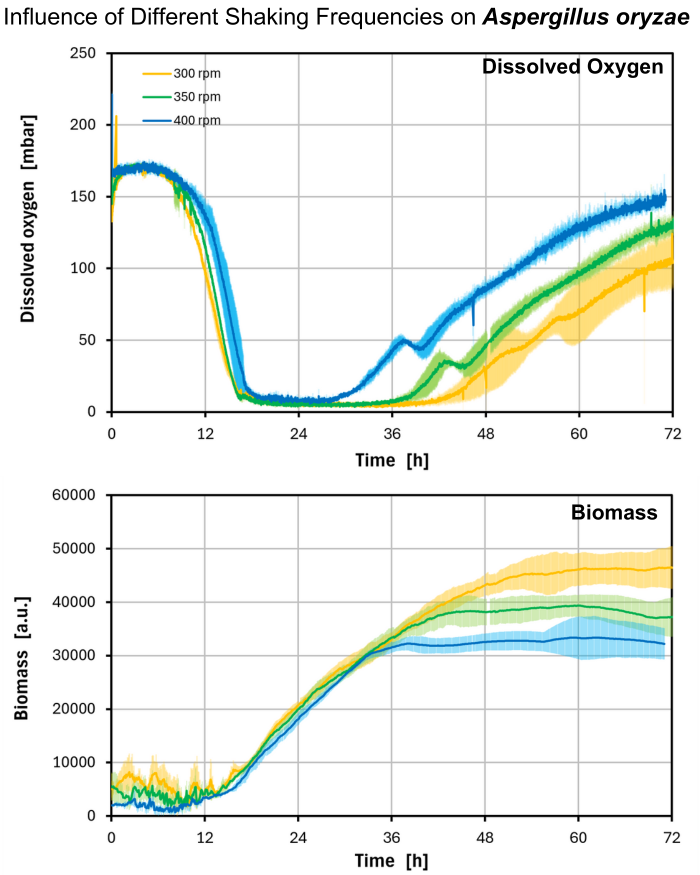
Dissolved oxygen levels [mbar] or biomass [a.u.], respectively, monitored for A. oryzae cultures shaken at three different shaking speeds: 300 rpm, 350 rpm, or 400 rpm. Dissolved oxygen levels were monitored with the help of the DO Sensor Pills and the Multiparameter Sensor (MPS). The same MPS simultaneously monitored biomass. Displayed are average values and standard deviations of three individual measurements. The data was visualized in real-time and analyzed with the DOTS Software.
Diameters of mycelium aggregates were analyzed offline and size differences between different shaking conditions were revealed:
300 rpm: ~ 0,905 mm ∅350 rpm: ~ 0,681 mm ∅
400 rpm: ~ 0,584 mm ∅

Materials & Methods
250 mL shake flasks were filled with 80 mL of DPY medium which was innoculated with 10^6 spores/ mL of the A. oryzae species. The pH was set at 5.5 - 6. At a temperature of 30°C and a shaking diameter of 1.9 cm, three different shaking speeds were applied throughout the fermentation: 300 rpm, 350 rpm, and 400 rpm.
Dissolved oxygen and biomass were monitored online with the DOTS Platform (DO Sensor Pills and Multiparameter Sensor) in triplicates. Every 24 hours, a sample was taken for further analysis. The size of cell aggregates was measured and documented.
Conclusion
As anticipated, increasing the shaking speed resulted in improved dissolved oxygen availability and reduced the duration of oxygen limitation for the fungal cells. Moreover, the varying shaking conditions visibly influenced cell morphology. Measurement of mycelium aggregates revealed that the culture shaken at 300 rpm (the slowest speed) exhibited the largest aggregates (0.905 mm ∅), while higher shaking speeds yielded progressively smaller mycelium aggregates (0.681 mm ∅ for 350 rpm and 0.584 mm ∅ for 400 rpm).
Despite the challenges inherent in monitoring fermentations during the cultivation of filamentous fungi species, dissolved oxygen and biomass levels were continuously and reliably monitored, providing real-time visualization through the DOTS Platform. For the first time, researchers conducting this study could observe these parameters online, enabling conclusions drawn from authentic datasets rather than assumptions. Through the optimization of their process via higher shaking speeds, they enhanced cell viability and protein production, preparing them for the upscaling of their process to the bioreactor.
The study underscores that, even with filamentous fungi species cultivated in shake flasks, acquiring high-density data on critical parameters becomes easily achievable through the adoption of state-of-the-art bioprocessing sensors.
Source
Hochschule Niederrhein, Professor Michaela Wagner. Experiments were conducted by Janina Koprionik and Athanasios Georgoulis.
Want Results Like These?
We will work with you on a solution that works best for your application.
From Estimation To High-Resolution Growth Curves


Customer Success Stories
.png)
-Kitana Manivone Kaiphanliam (Washington State University)
