Data Spotlight
Optimization of Glucose Oxidase Production in Pichia pastoris Using the Multiparameter Sensor (MPS) and Liquid Injection System (LIS)
Background
Pichia pastoris, this methylotrophic yeast species has become a basic tool for the biotechnological production of recombinant proteins. It represents one of the best choices for industrial applications due to the several advantages associated with the organism such as:
- It is suitable for large-scale protein production since it has the potential to grow in high cell densities on very simple and inexpensive media. This makes the production of large amounts of recombinant proteins possible at relatively cheap prices.
- P. pastoris uses methanol as a carbon source, allowing for very controlled expression of proteins when partnered with a strong promoter such as AOX1. The properties include low background expression and higher yields.
- In addition to genetic stability and the relative ease of genetic manipulation in yeast, it is possible to obtain, within this organism, strains tailored to the requirements of manufacturing systems installed.
This work uses expressed glucose oxidase, an enzyme with considerable applications in the food and pharmaceutical industries, in a genetically modified Pichia pastoris for optimum production. The study aimed to show that P. pastoris could be the platform and test various growth behaviors of different constructs (the PaGox variants) in the Pichiapink strain, which can go a long way in improving yield and the production process. To do so, optimum growing conditions would have to be set, and advanced bioprocess monitoring and control systems installed.
Results
Materials & Methods
A Pichiapink strain 1 culture was prepared in 25 mL of BMGY medium, using glycerol as the carbon source for biomass production. The cultivation occurred in a 250 mL Erlenmeyer flask at a filling volume of 10% at controlled temperature and shaking conditions of 24°C and 250 rpm, respectively.
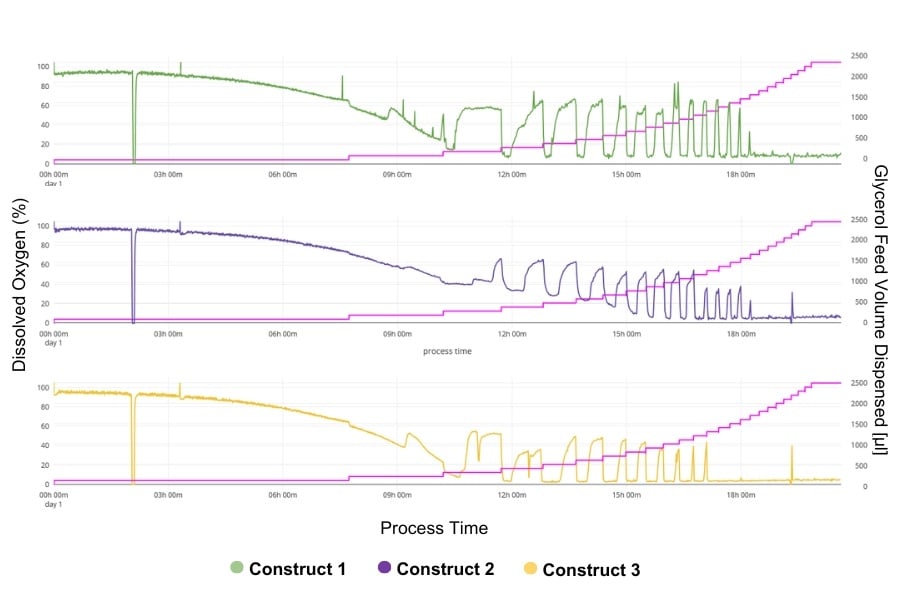
Feeding methanol into the culture from 20% to 40% via a Liquid Injection System (LIS) induced a metabolic shift to protein production. The dissolved oxygen was measured continuously by adding a DO sensor pill in the flask positioned above the Multi-Parameter Sensor (MPS).
The collected data was analyzed on the DOTS Platform, allowing the process to be tracked. These optimized conditions were first applied to Pichiapink (Construct 1) and then to Constructs 2 and 3. The initial batch phase established baseline conditions before switching to a fed-batch phase using methanol as an inducer of the AOX1 promoter driven expression of glucose oxidase.
Conclusion
The optimization process led to significant improvements in glucose oxidase production:
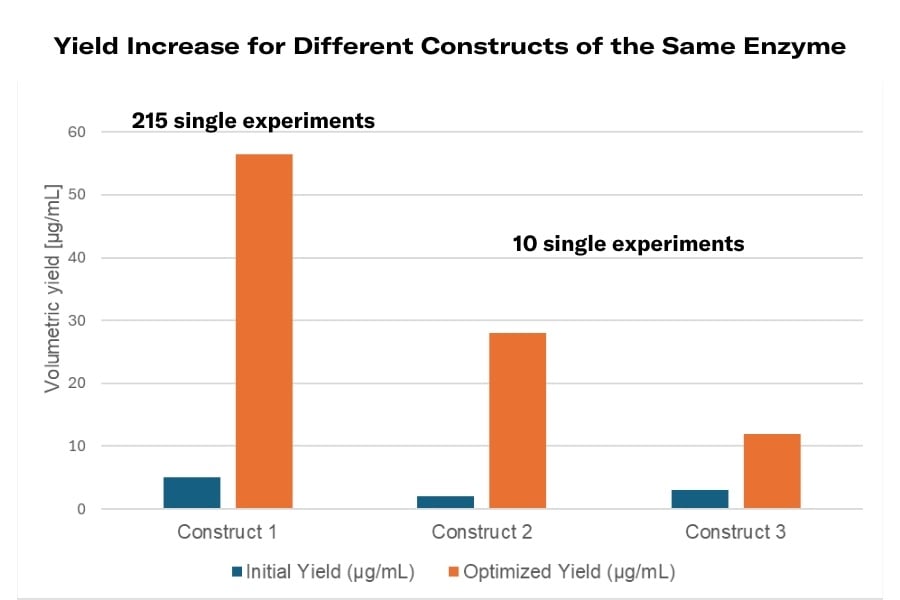
- Increased Yield: The glucose oxidase was produced by Pichia pastoris Construct 1 up to 56.5 µg/mL after the transition from the batch to the fed-batch phase. The highest peak yield achieved was after a series of 215 single experiments.
- Constructs-Specific Behavior: Using these conditions in other glucose oxidase variants, it could be realized that each construct responded differently. Significant differences relating to oxygen limitation phases and adaptation times for the various constructs were demonstrated that clearly show the necessity for tailored optimization strategies.
- Efficiency in Optimization: In the process optimization, remarkable efficiency was shown by the usage of MPS (Multi-parameters platform), LIS (Liquid Injection System), and the PID controller. For instance, whereas 215 experiments had to be conducted to achieve the optimal yield in Construct 1, a similar level of optimization in Construct 2 was obtained after only 10 experiments, producing half of the maximum production in Construct 1.
Have questions about your application?
Let’s work together to find a solution that works best for you.
From Estimation To High-Resolution Growth Curves


Customer Success Stories
.png)
-Kitana Manivone Kaiphanliam (Washington State University)
