Data Spotlight
Impact of Starter Culture Conditions on Strain Growth
Background
The preculture media and adaptation time of the microorganism are critical factors that influence the efficiency of a bioprocess, including the seed train scale-up. The composition of the culture media, its nutrients, pH, and the media's freshness directly impact the microorganisms' readiness state. When cells are transferred to a new media, their ability to quickly adapt which known as the adaptation time, is crucial for maintaining productivity, when is the time to shift from biomass production to the product formation, since less adaptation times mean that cells can swiftly resume exponential growth, optimizing biomass production and product yield.
These effects are highlighted during the seed train scale-up as cells are progressively transferred to larger bioreactors. Each scale-up stage requires careful media design to ensure smooth transitions and minimize adaptation times by closely matching the conditions of each stage.
Results
Materials & Methods
A Pichiapink strain was prepared in three different precultures and grown in 250 mL flasks with a filling volume of 10%. A Dissolved Oxygen Pill was added for the dissolved oxygen measurements. These cultures were shaken at 24°C and 250 rpm as follows:
- Old Culture: Kept over the weekend, reinoculated on Monday, and grown for two days (stationary phase).
- 2-Day Culture: Inoculated from cryo culture and grown for two days (stationary phase).
- 1-Day Culture: This was inoculated from a 2-day culture and grown for one day (exponential growth phase).
Glycerol was added as a fed batch (1% v/v) for 24 h of exponential feeding for biomass production. After 4 hours of starved culture, when DO recover to 80%, 0.5% methanol was added via the Liquid Injection System (LIS) for a metabolic shift toward protein production. The dissolved oxygen (DO) was measured by adding DO Sensor Pill to the flasks settled above Multi-Parameter Sensors (MPS) and the data analyzed by the DOTS Platform.
Conclusion
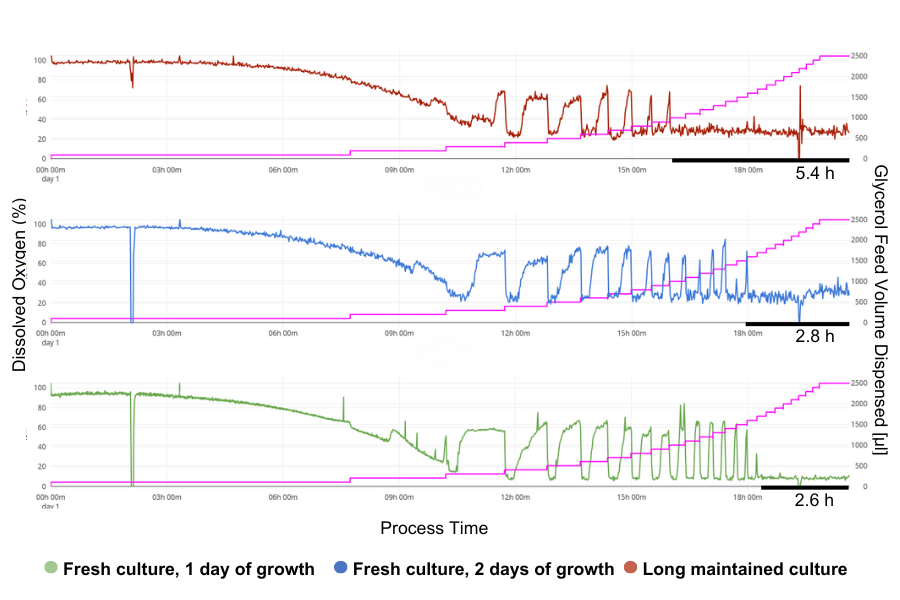
The three cultures show significantly different results in the response to glycerol feeding.
The oldest culture showed the most repressed response of the glycerol, where the oxygen-limited phase lasted 5.4 hours. This duration dropped to 2.8 hours in the 2-day culture and 2.6 hours in the 1-day culture, showing a progressively faster response with decreasing the age of the culture.
The 1-Day Culture shows the most rapid adaptation to the glycerol feed, as indicated by the DO signal. It quickly recovers after each glycerol shot and maintains DO recovery for the longest period during feeding, only reaching oxygen limitation at the end of the exponential feed phase. This quick adaptation in the freshest culture assures the significance of initial preculture adjustments, particularly in terms of methanol adaptation. In addition to the proper adaptation to methanol is crucial, as differences in adaptation time can greatly impact when to start subsequent feeding, because starting too early could lead to excessive methanol intake, which may have toxic effects.
These results confirm the importance of monitoring and controlling bioprocessing to reach high-quality outputs. The integration of the Multiparameter Sensor (MPS) for automated, online measurement of biomass in shake flasks and the DO Sensor Pill for online DO monitoring, while the Liquid Injection System (LIS) for automated liquid feeding means optimal parameter control is assured. This combination makes it possible to handle culture conditions very accurately, ultimately allowing better metabolic performance and overall process efficiency.
Have questions about your application?
Let’s work together to find a solution that works best for you.
From Estimation To High-Resolution Growth Curves


Customer Success Stories
.png)
-Kitana Manivone Kaiphanliam (Washington State University)
