
Microbial Screening In Shake Flasks
Finding The Needle In The Haystack
With the ability to conduct multiple experiments simultaneously, shake flasks are a researcher's best friend when it comes to efficient and versatile microbial screening experiments.
From strain screening in strain development, seeking that perfect production strain, to media screening, where we optimize the culture medium for enhanced results – microbial screening opens a wide array of possibilities.
While high-throughput capabilities are commonly associated with screening, the significance of scale-up feasibilities is recognized more and more as well. Striking a thoughtful equilibrium between these two attributes becomes an indispensable asset for successful screening assays.
Researchers use versatile screening techniques to make sure the best strain is selected and fermentation conditions are ideally fine-tuned for the desired experiment outcome. Microbial screening is therefore a pivotal cornerstone for the success of most bioprocesses.
Diversity in Biology
From microorganisms like bacteria, fungi, algae, and protozoa to complex organisms like plants and animals, the biological world presents an astonishing diversity. Microorganisms, in particular, are incredibly varied, with billions of species, and hold significant applications in medicine, agriculture, biotechnology, and environmental conservation. We utilize these cells to produce antibiotics, enzymes, biofuels, and more, impacting our daily lives. Scientists seek to understand and classify the diverse geno- and phenotypes among microorganisms. Beyond species diversity, interactions between microorganisms and different media compositions result in varying product formations and performances in biotechnological applications. Microbial screening helps to navigate through the vast landscape of diversity, enabling researchers to find clarity and make informed decisions.
Defining the Optimization Target
The first step of every screening process is to define the screening criteria. To clarify one optimization target is important for effectively guiding the selection and evaluation of microorganisms or experimental conditions, ensuring that the screening process yields relevant results.
Common optimization targets in biotechnology:
-
Higher product titers
-
Better space-time yield: More product in less time and space
-
Reduction of by-product formation
-
Efficient carbon source usage
-
Increased level of resistency towards certain chemical compounds
Multifaceted Screening Areas
There exist multiple pathways to accomplish a desired optimization target. These may include screenings for the best production strain, for ideal media compositions or fermentation conditions, and the right timing of workflows, such as induction time points for promotor activation.

Strain Engineering
Identifying the ideal strain for a bioprocess represents a crucial initial step in process development, significantly influencing its overall success. Advanced technologies, such as metabolic engineering or adaptive evolution, are employed to introduce genetic variations into microbial strains, aiming to enhance specific attributes like productivity, substrate preferences, or resistances. Various methods are used to screen for the ideal production strain and assess the impact of genetic modifications on its actual performance. These methods include proteomic analyses, enzyme activity assays, stress tests, and evaluation of fermentation outputs, such as biomass formation or product yields.
📃 Read the sbi Success Story with Clariant about Strain Screening under Fed-batch Conditions

Media Optimization
Media optimization is a critical aspect of bioprocess development, aimed at maximizing the growth and productivity of microbial cultures. The selection and formulation of an appropriate growth medium can significantly impact the performance of the desired microorganism. Through systematic adjustments of nutrient compositions, researchers can fine-tune the medium to suit the specific needs of the microbial strain. By employing cutting-edge techniques and rigorous experimentation, bioprocess engineers can unlock the full potential of microorganisms, leading to more efficient and cost-effective bioprocessing.
📃 Read the sbi Success Story with Bond Pet Foods about Media Optimization

Fermentation Conditions
Fermentation conditions play a pivotal role in bioprocess optimization, directly impacting the efficiency of microbial fermentations. The manipulation of environmental parameters, such as temperature, pH, dissolved oxygen, agitation speed, or vessel type can significantly influence the growth and metabolic activity of the microorganism. Screening for and selecting the optimal fermentation conditions is often a complex task, requiring a balance between microbial physiology and technical feasibility. Advanced monitoring and control systems are employed to maintain precise conditions throughout the fermentation process, ensuring reproducibility and scalability.
📃 See how you can monitor and optimize conditions in your fermentation using sbi’s smart shake flask technology

Timing of Workflows
The timing of workflows in bioprocesses significantly influences overall efficiency and success and is therefore also a subject of screening. Proper coordination of steps like inoculation, nutrient addition, and product harvest impacts yield and quality of the final product. Careful design ensures optimal resource utilization, reduced processing time, and enhanced microbial productivity, while strategic timing of specific events like promotor induction triggers specific metabolic pathways, leading to desired metabolite and protein accumulation. Time points of promotor induction, for instance, are strategically chosen by induction screenings to activate the expression of target genes precisely when needed.
📃 Read the sbi Success Story about Induction Screening with Pichia pastoris
Most Common Parameters in Screening
In screening experiments, scientists consider different parameters based on their specific optimization targets. Monitoring multiple parameters is often beneficial for comprehensive assessment and informed decision-making during the screening process.
- Biomass
- pH
- Dissolved Oxygen
- Product Titer
- Enzyme Activity
- Resistencies
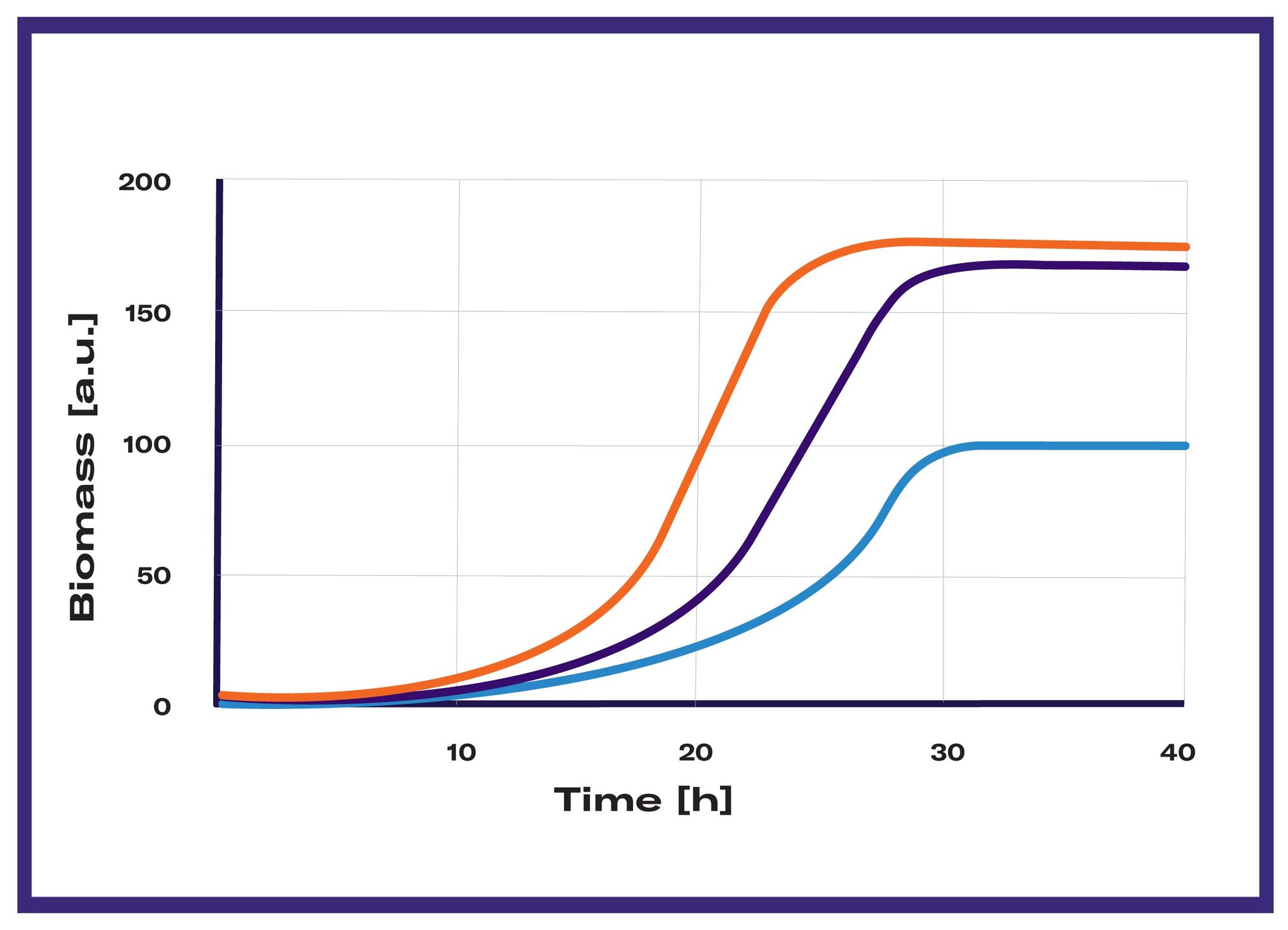
In the majority of screening experiments, the cell growth or total biomass of a culture is a critical parameter. Even when not the primary point of interest, it is frequently assessed to evaluate the impact of various factors on the growth rate and overall health of the microorganism. When product titers are the focus of screening, they are often normalized relative to cell density for better comparability. Growth tests are therefore commonly conducted in the form of continuous biomass monitoring in liquid cultures, drop tests on agar plates, or cell dry weight measurements at the end of the fermentation.
Learn about the Monitoring of multiple parameters with the MPS.
Monitoring dissolved oxygen levels in aerobic processes is critical to ensuring sufficient oxygen is available for aerobic organisms, as it directly influences cellular respiration and growth rates. It also provides valuable information about the system's efficiency and potential limitations related to oxygen transfer or utilization.
The term product titer refers to the concentration or yield of the desired product generated during a process. Monitoring and optimizing product titers are essential for enhancing productivity and ensuring the efficiency of the production system, ultimately contributing to the development of economically viable processes.
%20(2)-1.png)
Enzyme activity is a critical parameter in screening experiments involving biocatalysts, as it measures the speed at which an enzyme extract catalyzes a specific reaction and is used for assessing enzyme efficiency. Scientists frequently aim to optimize their bioprocesses to increase the yield of active enzymes, employing activity tests as a reliable assessment method.

The ability of organisms or cells to withstand specific stressors is a subject of intensive investigations and thus screening procedures. These stressors can include exposure to antibiotics, toxins, extreme temperatures or salinity, or other adverse conditions. Understanding resistances is crucial for engineering robust industrial microorganisms. Screening experiments that assess an organism's response to different concentrations of a toxic substrate are also known as toxicity tests.

In the majority of screening experiments, the cell growth or total biomass of a culture is a critical parameter. Even when not the primary point of interest, it is frequently assessed to evaluate the impact of various factors on the growth rate and overall health of the microorganism. When product titers are the focus of screening, they are often normalized relative to cell density for better comparability. Growth tests are therefore commonly conducted in the form of continuous biomass monitoring in liquid cultures, drop tests on agar plates, or cell dry weight measurements at the end of the fermentation.
Learn about the Monitoring of multiple parameters with the MPS.


Monitoring dissolved oxygen levels in aerobic processes is critical to ensuring sufficient oxygen is available for aerobic organisms, as it directly influences cellular respiration and growth rates. It also provides valuable information about the system's efficiency and potential limitations related to oxygen transfer or utilization.

The term product titer refers to the concentration or yield of the desired product generated during a process. Monitoring and optimizing product titers are essential for enhancing productivity and ensuring the efficiency of the production system, ultimately contributing to the development of economically viable processes.
%20(2)-1.png)
Enzyme activity is a critical parameter in screening experiments involving biocatalysts, as it measures the speed at which an enzyme extract catalyzes a specific reaction and is used for assessing enzyme efficiency. Scientists frequently aim to optimize their bioprocesses to increase the yield of active enzymes, employing activity tests as a reliable assessment method.

The ability of organisms or cells to withstand specific stressors is a subject of intensive investigations and thus screening procedures. These stressors can include exposure to antibiotics, toxins, extreme temperatures or salinity, or other adverse conditions. Understanding resistances is crucial for engineering robust industrial microorganisms. Screening experiments that assess an organism's response to different concentrations of a toxic substrate are also known as toxicity tests.
Vessel Types for Screening
Different vessel types are used in microbial screening experiments to accommodate various experimental scales and optimize conditions for high-throughput screening or in-depth characterization of biological processes.
Solid Medium
Test Tubes
Shake Flasks
Bioreactors
Parallel Bioreactor Systems
Screening in Shake Flasks - The Advantages
The ease-of-use and cost-effectiveness make shake flasks an ideal choice for researchers conducting multiple small-scale experiments concurrently, particularly for microbial screening assays. Unlike very small reaction vessels like test tubes or microtiter plates, shake flasks offer superior cultivation conditions, ensuring enhanced aeration and media mixing, which prove highly beneficial for the growth of microorganisms. The larger culture volume in these vessels permits additional sampling, which is not feasible in smaller vessel types due to the smaller volume. While they may not perfectly mimic large-scale bioreactor conditions, the feasibility of scaling up experiments is improved, and modern sensing and control options further enhance data transferability from shake flasks to bioreactors.
Key Benefits
- Easy to use, also for untrained labworkers
- High-throughput compared to larger vessel types
- Flexible scalability of experiment conditions by adding or removing flasks
- Sufficient reaction volume for additional analyses
- Cost-efficient screening procedures
- Accessible to most laboratories
High-throughput vs. Scale-up Feasibility
A high-throughput is a sought-after characteristic in screening assays as it enables researchers to process a large number of samples quickly. However, when high-throughput is achieved through the use of extremely small reaction volumes it can lead to challenges in accurately translating results to a larger scale. Increasing the reaction volumes can enhance the reproducibility and reliability of the results, ensuring a smoother transition to larger-scale applications. Finding the right balance between throughput and volume becomes essential for obtaining meaningful data that is both efficient and transferable.
The Shake Flask Bioreactor
At sbi, we believe that bioreactor-like shake flasks have the potential to become the ideal cultivation vessel for microbial screening experiments. The ease-of-use of shake flasks allows individuals without specialized training to set up and conduct experiments flexibly with a higher throughput when compared to bioreactors. The DOTS platform provides cutting-edge sensors and actuators designed for shake flasks, introducing bioreactor-like control. This innovation empowers researchers to conduct bioreactor-like experiments within this widely popular vessel type, overcoming previous barriers on data monitoring and control options.
Hear What Our Customers Say
Bond Pet Foods Saves Time and Money in Their Media Optimization Process with the Cell Growth Quantifier
"The real power of the CGQ is being able to sample at specific points of interest based on the continuous, high-resolution data. Otherwise, with manual sampling, it is just a shot in the dark. You never really know where in the growth curve the organism is."
Raul Reveles, Senior Bioprocess Engineer at Bond Pet Foods

