Lack of Control
- Lacks bioreactor control options for e.g., pH control or fed-batch
- Leads to inconsistencies in scale-up between shake flask and bioreactors
Open a whole new world of bioprocess development when you use LIS to perform automatic feeding in shake flasks. Simply assemble the components, define the experiment parameters, fill the cartridge with the feeding liquid, and start feeding.
Key Features
Benefits
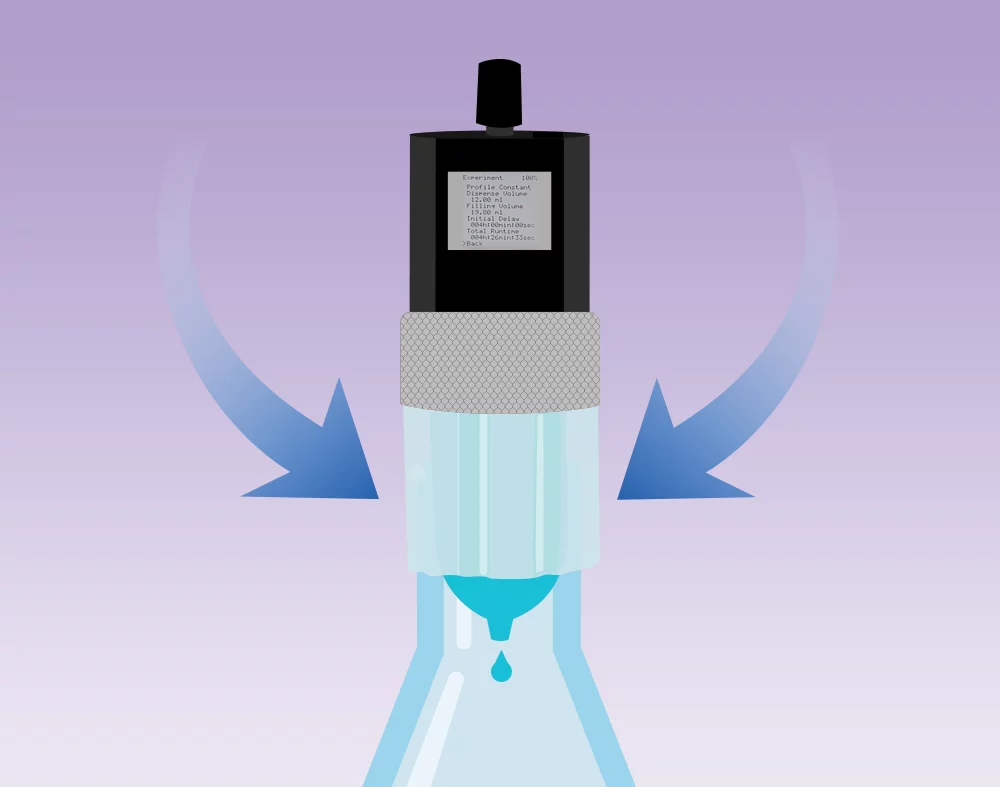
In order to dispense a defined amount of liquid from the cartridge, the drive pumps air through a sterile filter into the cartridge according to the feeding profile.
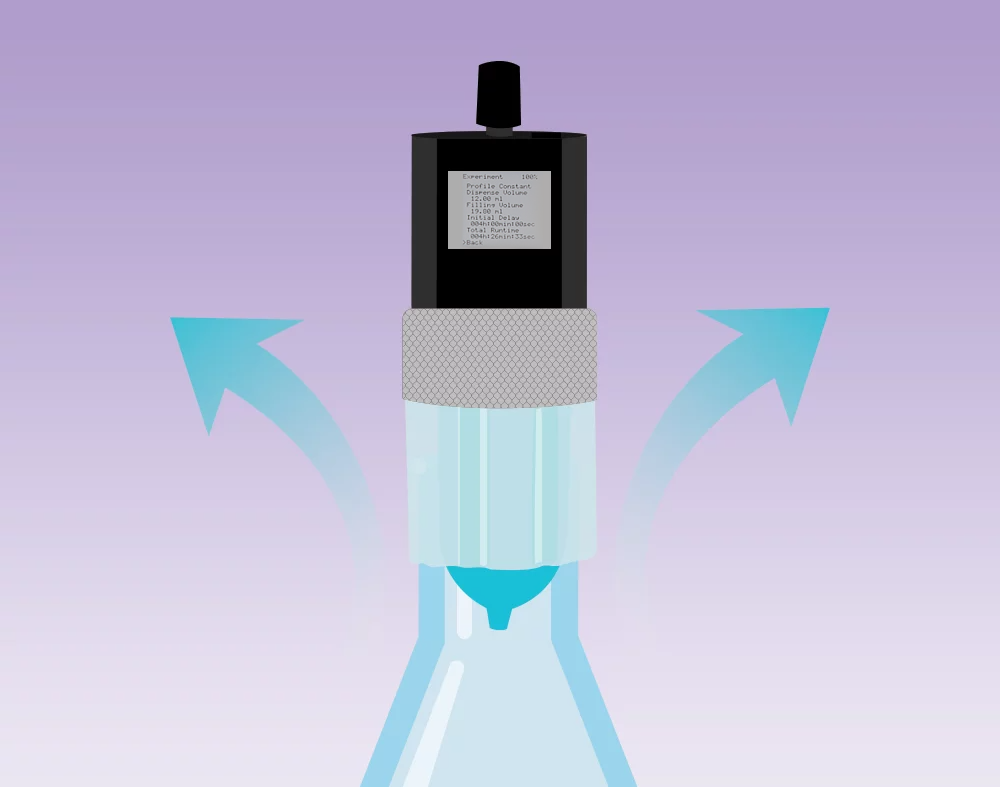
After the dispense step, the drive rebuilds negative pressure to keep the remaining liquid in the cartridge.
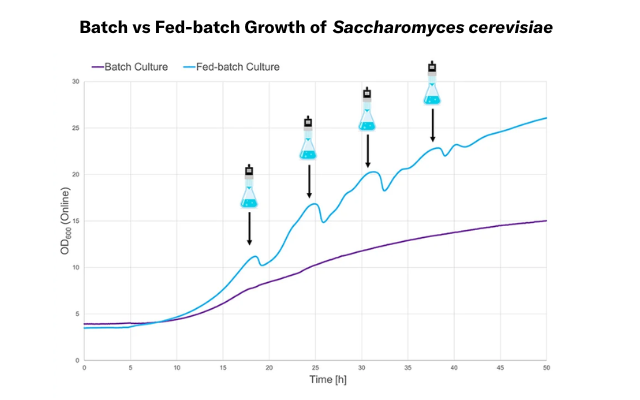
With LIS, typical bioreactor processes, like fed-batch cultivations, can be recreated in shake flasks. Fed-batch cultures support the growth of S. cerevisiae on potato waste because the concentration of growth inhibitors in the medium can be controlled. With the LIS, these process conditions could be imitated on a shake flask level.
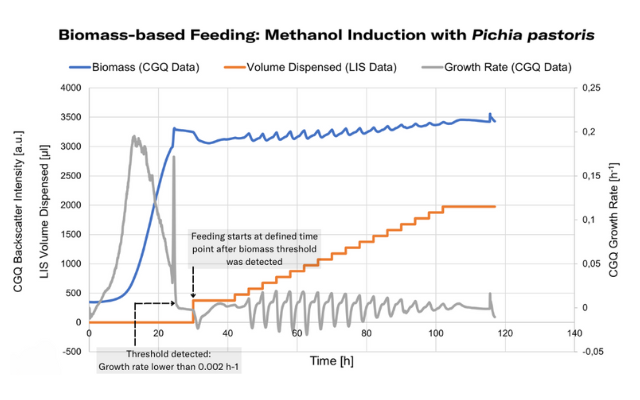
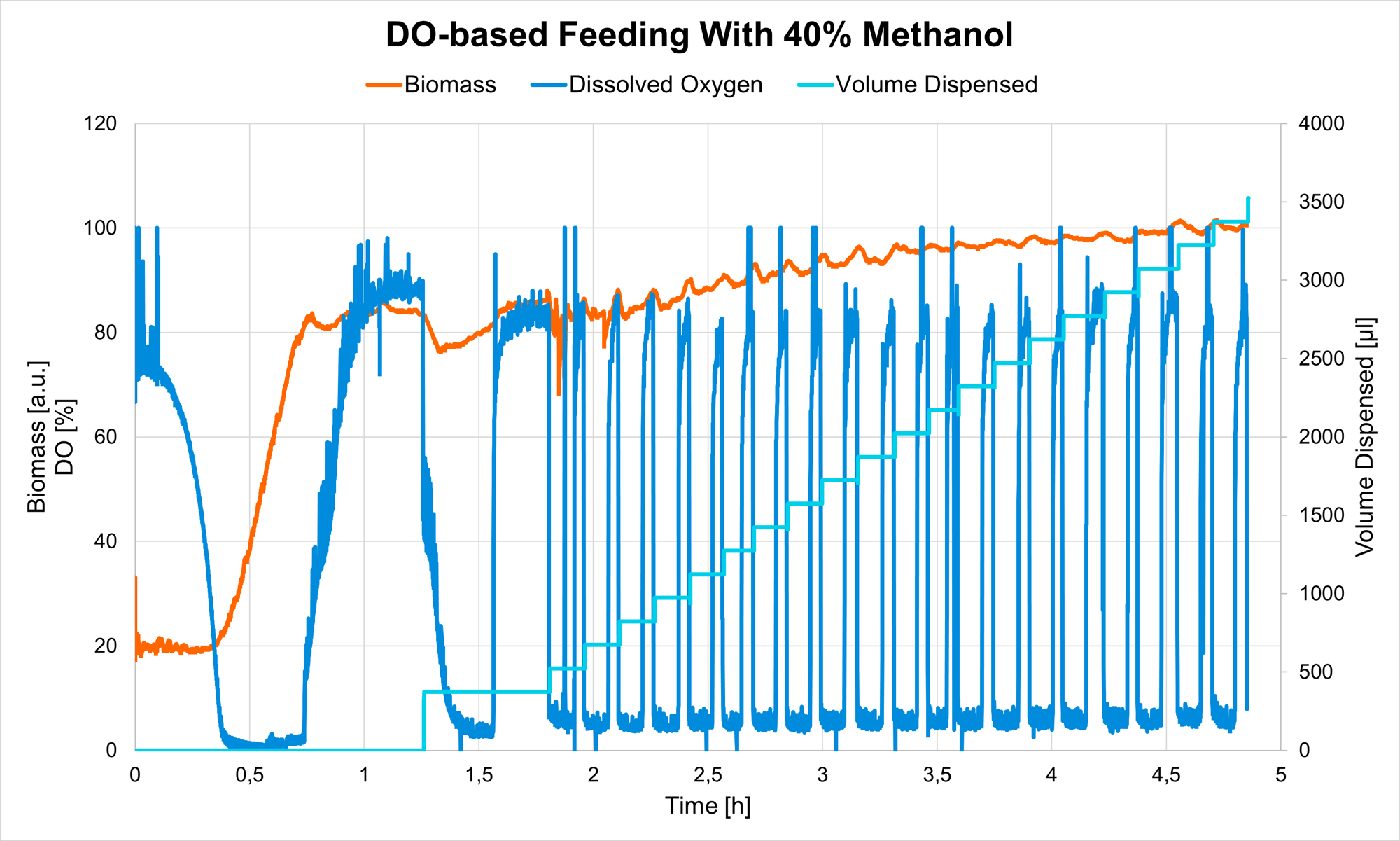
Enable feedback-controlled feeding in your shake flask experiments when you connect LIS with reliable biomass monitoring sensors such as the Cell Growth Quantifier (CGQ), or Multiparameter Sensor (MPS), and the DOTS Software. Biomass-based feeding can be used to initiate methanol induction at the optimal time - once cell growth on the primary substrate (glycerol) is complete and sufficient biomass was generated, indicated by a reduction in growth rate.
With a DOTS-integrated controller, the methanol feed (carried out with the Liquid Injection System) was adjusted to start repeatedly, always when the DO level, monitored by DO Sensor Pills, reached a preset threshold. By using this feature, methanol was always fed as soon as the cells recovered from the previous shot, enabling ideal cell viability, while keeping promotor activity constant. Biomass was monitored additionally, with the Multiparameter Sensor (MPS).
The drive is a programmable miniature pump that controls when and how much liquid is fed from the cartridge into the flask.

The cartridge is a pre-sterilized, ready-to-use container that fits on top of the shake flask. It can be filled with up to 25 mL of any type of liquid and acts as a reservoir during the feeding experiment.
The DOTS Software allows you to wirelessly control and monitor your feeding experiments. Create individual feeding profiles, define feeding parameters, and monitor the expensed liquid volume over time. By combining other DOTS integrated sensors, parameter-based feeding can be realized.




Use the interconnectivity of sensors (CGQ) and actuators (LIS) within the new DOTS Software to enable feedback controlled feeding in your shake flask experiments. Set up your injection strategy based on your biomass threshold or growth rate and start feeding based on the specific needs of your culture.
The LIS is designed to fit any 38 mm straight neck Erlenmeyer shake flask.
LIS has been successfully tested with a broad variety of liquids. Our team of application scientists will work with you to ensure that the liquid and concentration needed for your particular application can be used with our feeding system.
Sugars
Alcohols
Glycerol
Acids, bases, and inductors (e.g., IPTG)
Antifoam
No. The LIS is designed to hold one liquid at a time in its single reservoir (cartridge). To switch to a different feeding solution, the cartridge must be replaced, which requires setting up a new LIS assembly (drive + cartridge) and restarting the feeding mid-run. It is not possible to feed two liquids at the same time with LIS.
No. The LIS setup closes your shake flask in a similar way as an aluminium cap and allows for gas exchange. Check out this data set comparing the evaporation rates of shake flasks with different closing types. It shows that a LIS closing does not limit gas transfer in comparison to conventional shake flask closings.
The cartridges are single-use products and we do not recommend to reuse them.
The LIS offers several different feeding profiles, such as single- or multi-shot injections, constant feeding, or exponential feeding, providing customized solutions for almost any experiment. Learn more on our Application Page. The cartridge has a maximum capacity of 25 mL. For best results, we recommend a filling volume between 8 and 20 mL. The smallest possible feeding volume is 100 µL. Hence, a single shot can range from 100 µL and the maximum feeding rate is 1 mL/ min.
If the setup is not carried out correctly, it may not be completely sealed, which can result in leaks or dripping. Therefore, it is essential to handle the setup carefully and follow every step in the procedure. For a detailed, step-by-step demonstration, please refer to our How to video under Resources (on this tabber).
Some common mistakes that can lead to leakage include:
1) Forgetting to auto-offset the pressure of the Drive. Always ensure the pressure is auto-offset to 0 before starting the experiment; otherwise, the setup is unlikely to be leak-proof. This auto-offset must be done with the Drive alone, without cartridges or filter added.
2) Omitting the sterile filter. Each cartridge comes with a sterile filter that must be attached. Do not forget to include it.
3) Removing the luer-plug too early. Always prepare the drive and wait for the LIS Drive to stop pumping before removing the luer-plug. This allows the pump to create an underpressure that keeps the liquid in place.
4) Not filling the cartridge nozzle (LIS outlet) completely. Ensure the tip is filled completely with liquid and no air bubble is present at the bottom of the cartridge before starting an experiment.
5) Using the wrong liquid parameters that specify the liquid in use. When using parameters that differ (sometimes even slightly) from the used liquid, dripping is possible.
6) Stating the wrong filling volume: Filling the Cartridge with more or less liquid than stated in the software can lead to dripping.
LIS has been successfully tested with a broad variety of liquids. These include (but are not limited to) a broad range of sugars, alcohols, acids, and bases. The liquid type needs to be specified in the Software- select the option that most closely matches your liquid. If you cannot find your liquid in your list or are unsure if it resembles the liquids from the list sufficiently, please contact our support team.
Yes, but feeding volatile liquids introduces some challenges. Volatile liquids (e.g., highly concentrated methanol) can evaporate continuously. This causes the Drive to pump more frequently, which may reduce the negative pressure and potentially lead to dripping. There are certain countermeasures that help to prevent these. Please, reach out to our service team for advices.
It is not recommended to remove the LIS setup during the experiment. If you plan to sample during the experiment, we recommend to use a 2-neck shake flask and use the second neck to withdraw samples. (Be careful not to tilt the flask excessively, as this may wet the filter.)
The LIS Drive must not be autoclaved, as this will destroy the device. It should also never come into contact with water. If the Drive got dirty, clean it carefully using a slightly wet wipe (avoiding the sensitive white outlet nozzle) and then dry it thoroughly. The cartridges and filters are single-use components. They are pre-sterilized with ethylene oxide, packaged sterile, and therefore do not require any additional cleaning or sterilization.
The LIS is designed to fit any 38 mm straight neck Erlenmeyer shake flask.
LIS has been successfully tested with a broad variety of liquids. Our team of application scientists will work with you to ensure that the liquid and concentration needed for your particular application can be used with our feeding system.
Sugars
Alcohols
Glycerol
Acids, bases, and inductors (e.g., IPTG)
Antifoam
No. The LIS is designed to hold one liquid at a time in its single reservoir (cartridge). To switch to a different feeding solution, the cartridge must be replaced, which requires setting up a new LIS assembly (drive + cartridge) and restarting the feeding mid-run. It is not possible to feed two liquids at the same time with LIS.
No. The LIS setup closes your shake flask in a similar way as an aluminium cap and allows for gas exchange. Check out this data set comparing the evaporation rates of shake flasks with different closing types. It shows that a LIS closing does not limit gas transfer in comparison to conventional shake flask closings.
The cartridges are single-use products and we do not recommend to reuse them.
The LIS offers several different feeding profiles, such as single- or multi-shot injections, constant feeding, or exponential feeding, providing customized solutions for almost any experiment. Learn more on our Application Page. The cartridge has a maximum capacity of 25 mL. For best results, we recommend a filling volume between 8 and 20 mL. The smallest possible feeding volume is 100 µL. Hence, a single shot can range from 100 µL and the maximum feeding rate is 1 mL/ min.
If the setup is not carried out correctly, it may not be completely sealed, which can result in leaks or dripping. Therefore, it is essential to handle the setup carefully and follow every step in the procedure. For a detailed, step-by-step demonstration, please refer to our How to video under Resources (on this tabber).
Some common mistakes that can lead to leakage include:
1) Forgetting to auto-offset the pressure of the Drive. Always ensure the pressure is auto-offset to 0 before starting the experiment; otherwise, the setup is unlikely to be leak-proof. This auto-offset must be done with the Drive alone, without cartridges or filter added.
2) Omitting the sterile filter. Each cartridge comes with a sterile filter that must be attached. Do not forget to include it.
3) Removing the luer-plug too early. Always prepare the drive and wait for the LIS Drive to stop pumping before removing the luer-plug. This allows the pump to create an underpressure that keeps the liquid in place.
4) Not filling the cartridge nozzle (LIS outlet) completely. Ensure the tip is filled completely with liquid and no air bubble is present at the bottom of the cartridge before starting an experiment.
5) Using the wrong liquid parameters that specify the liquid in use. When using parameters that differ (sometimes even slightly) from the used liquid, dripping is possible.
6) Stating the wrong filling volume: Filling the Cartridge with more or less liquid than stated in the software can lead to dripping.
LIS has been successfully tested with a broad variety of liquids. These include (but are not limited to) a broad range of sugars, alcohols, acids, and bases. The liquid type needs to be specified in the Software- select the option that most closely matches your liquid. If you cannot find your liquid in your list or are unsure if it resembles the liquids from the list sufficiently, please contact our support team.
Yes, but feeding volatile liquids introduces some challenges. Volatile liquids (e.g., highly concentrated methanol) can evaporate continuously. This causes the Drive to pump more frequently, which may reduce the negative pressure and potentially lead to dripping. There are certain countermeasures that help to prevent these. Please, reach out to our service team for advices.
It is not recommended to remove the LIS setup during the experiment. If you plan to sample during the experiment, we recommend to use a 2-neck shake flask and use the second neck to withdraw samples. (Be careful not to tilt the flask excessively, as this may wet the filter.)
The LIS Drive must not be autoclaved, as this will destroy the device. It should also never come into contact with water. If the Drive got dirty, clean it carefully using a slightly wet wipe (avoiding the sensitive white outlet nozzle) and then dry it thoroughly. The cartridges and filters are single-use components. They are pre-sterilized with ethylene oxide, packaged sterile, and therefore do not require any additional cleaning or sterilization.
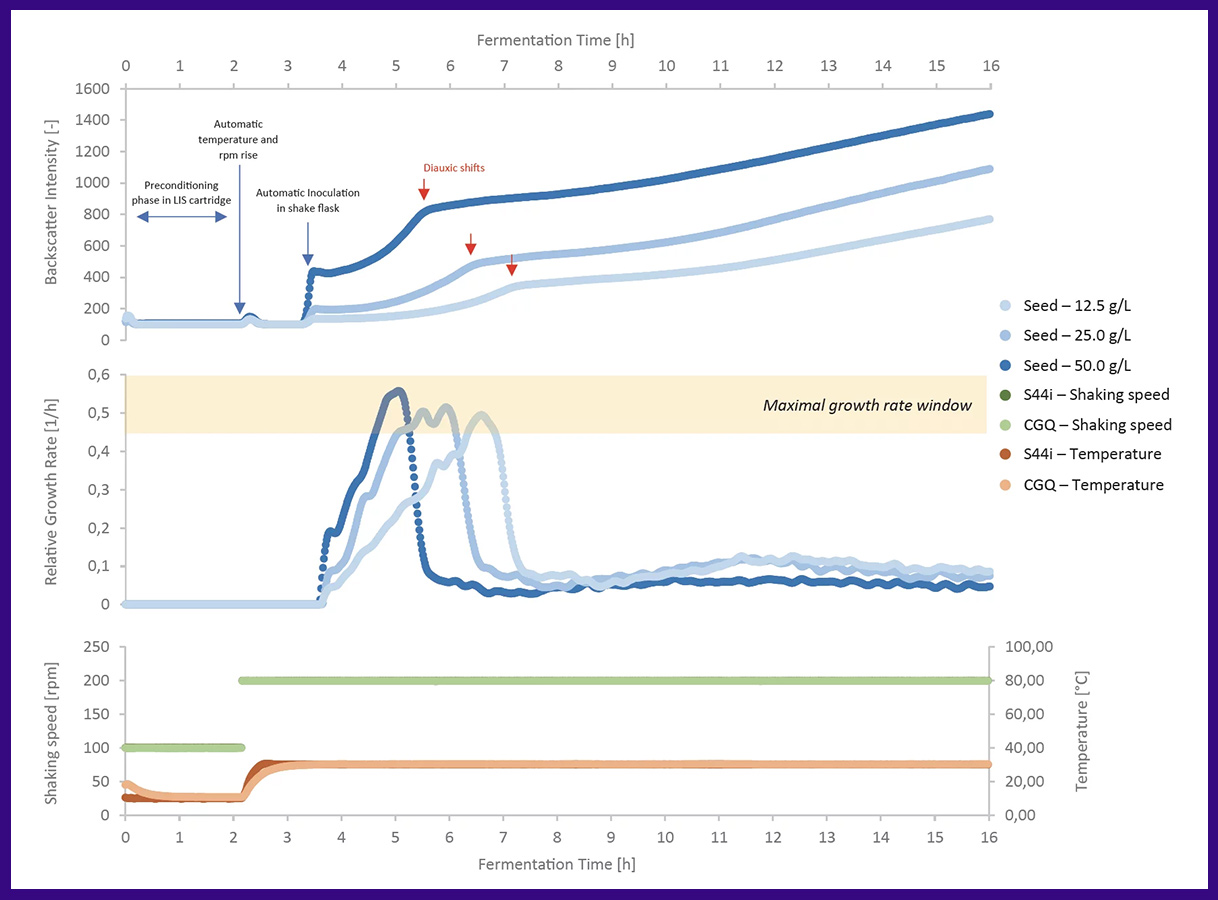
This application note illustrates the use of LIS in conjunction with the yeast S. cerevisiae to optimize bioreactor inoculum in shake flasks. After a period of preconditioning, the LIS automatically injects different seed biomass concentrations into a shake flask containing fresh YEPD. Cell growth is monitored in real-time to assess the reproducibility of environmental and growth phase conditions.
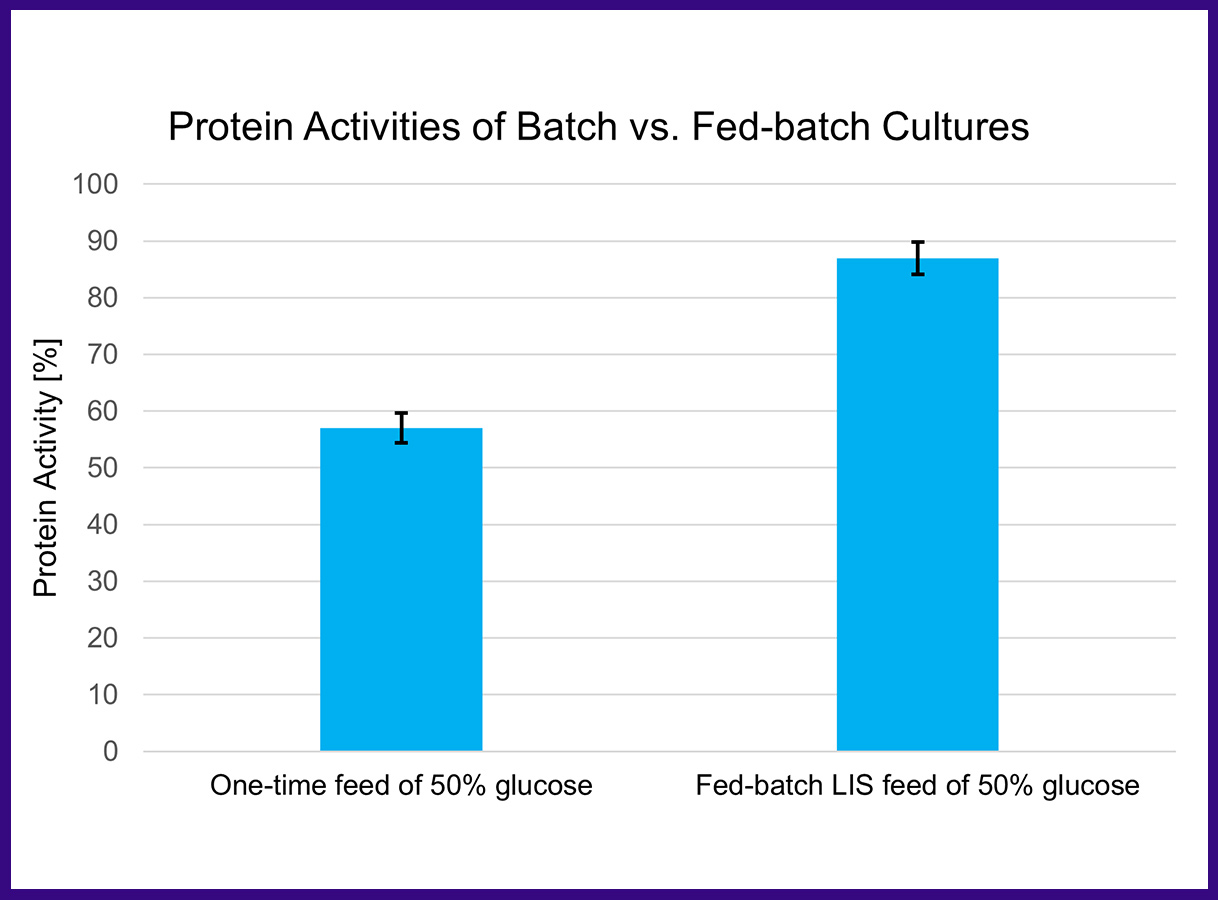
The batch condition has a great impact on the cell's metabolism which overshadows the strain's characteristic protein production behavior. A LIS facilitated fed-batch process showed a significant increase (30%) in active protein yields, compared to a batch process and is better suited to screen for the best production strain.
