Data Spotlight
Online Dissolved Oxygen Monitoring Uncovers Unique Metabolic Events in E.coli Fermentations
Background
Metabolic oscillations refer to the periodic or cyclic fluctuations in the levels of metabolites or response in certain parameters, such as dissolved oxygen, within a biological system. Metabolic oscillations have been previously reported in a number of bioprocesses, including E. coli fermentations. In this study, dissolved oxygen levels were closely monitored using DO Sensor Pills in shake flask fermentations of E. coli, grown in three distinct complex media compositions: Plain lysogeny broth (LB) medium, LB medium with 0.2% additional glucose, and terrific broth (TB) medium with 0.2% glycerol.
Notably, oscillations in dissolved oxygen levels were observed, distinct for each complex medium fermentation and consistent within duplicates of the same medium. This suggests that metabolic pathway fluctuations are influenced by media compositions.
Theories exist as to why these oscillations occur, with one prominent theory suggesting that occasional mismatches in amino acid profiles lead to rapid fluctuations in protein synthesis. When sugar is used for protein synthesis, oxygen is incorporated into cell protein rather than being respired to CO2, resulting in less oxygen usage and explaining the oscillations in oxygen levels.
While the exact metabolic reasons for the observed oscillations are not fully understood and require further study, the close, real-time monitoring of oxygen levels provides valuable insights into when these oscillations occur, significantly contributing to our understanding of this phenomenon.
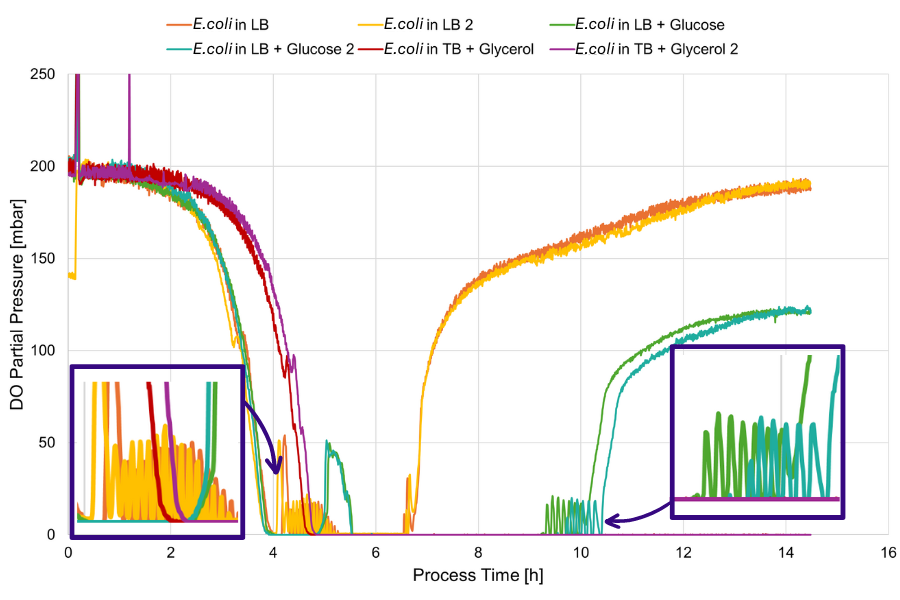
E.coli cultures were grown in three different complex media in duplicates: Plain LB medium (orange & yellow), LB + 0.2% additional glucose (green & turquoise), and TB + 0.2% glycerol (pink & purple). The same fermentation conditions were used for all cultures (37°C, 250 rpm, 10% filling volume in 250 mL shake flasks). Dissolved oxygen was closely monitored with the DO Sensor Pills and revealed different oxygen limitation times and detailed insights into distinct metabolic events specific for each medium.

Materials & Methods
250 mL shake flasks were filled with 10% of the respective medium:
1) Lysogeny broth (LB) with no additional sugar
2) LB medium with an addition of 0.2% glucose
3) Terrific broth (TB) medium with an addition of 0.2% glycerol
Main cultures were innoculated with E. coli cells from precultures to a starting OD of 0.04. All cultures were examined in duplicates. DO Sensor Pills were added (1 per each flask) and flasks were mounted on top of the Multiparameter Sensor. Real-time DO data was monitored and visualized with the DOTS Software. Data was exported to excel after the experiment. Cultivation took place at 37°C and a shaking speed of 250 rpm (at a shaker throw of 25 mm) for 14.5 hours.
Conclusion
Notably, the dissolved oxygen profile throughout E.coli fermentations grown in different media compositions differ significantly, whereas duplicates grown in the same medium and under the same conditions show precisely the same dissolved oxygen profile. The addition of 0.2 % glucose led to longer oxygen limitation, compared to the LB medium without additional glucose. The TB glycerol medium culture on the other hand went into oxygen limitation later, but didn't recover until the end of the fermentation. This shows that the different media compositions have a pronounced effect on oxygen consumption throughout the E.coli fermentation. Due to the high data density derived from dissolved oxygen measurements with DOTS DO Sensor Pills, more detailed effects of the media on oxygen levels could be observed in addition. These metabolic oscillations were detected as reocurring peaks in cultures grown in LB medium at distinct timepoints. Although the precise metabolic causes of the observed oscillations remain unclear and warrant additional research, the close, real-time monitoring of oxygen levels offers valuable insights into the timing of these oscillations, greatly supporting research in this field.
Source
Further reading on metabolic oscillations in E.coli fermentations: Andersen D.C., et al., 2001. Metabolic Oscillations in an E. coli Fermentation. DOI: 10.1002/bit.10018.
Interested in real-time dissolved oxygen monitoring in shake flasks? Check out this Application Page!
Want Results Like These?
We will work with you on a solution that works best for your application.
From Estimation To High-Resolution Growth Curves


Customer Success Stories
.png)
-Kitana Manivone Kaiphanliam (Washington State University)
